ZOFFANIES PTY LTD v FC of T
Members:RP Handley DP
Tribunal:
Administrative Appeals Tribunal
MEDIA NEUTRAL CITATION:
[2002] AATA 758
RP Handley
This matter concerns an application dated 20 September 2001 made by Zoffanies Pty Ltd (``the Applicant'') for a review of a decision of the Commissioner of Taxation (``the Respondent'') made on 26 July 2001 to disallow the Applicant's objection against an Income Tax Assessment dated 14 November 2000 for the Substituted Accounting Period ending on 30 September 1992 (in lieu of the year ending on 30 June 1992). The Applicant, a subsidiary company of Macquarie Bank Ltd (``MBL''), claimed it was entitled to an allowable deduction of $109,947 which was part of a loss of $20,720,962 (as adjusted) incurred by Macquarie Syndication (No 3) Pty Ltd (``MS3'') in that year of income (CT142).
2. The hearing in this matter took place in Sydney over a period of 3 weeks commencing on 5 August 2002. Lead Counsel for the Applicant was TF Bathurst, QC and for the Respondent was JR Sackar, QC. At the hearing, the evidence before the Tribunal comprised the documents produced pursuant to s 37 of the Administrative Appeals Tribunal Act 1975 (the ``T Documents'') together with the documents tendered by the parties. The following witnesses gave evidence: for the Applicant - Dr Robert Seamark, Dr Allan Robins, Mr Peter Hart, Dr John Smeaton, Mr Daniel Phillips, Dr Maurice Venning, Mr Robert Mooney, Mr Todd Morrill, and Professor Tom Smith; for the Respondent - Mr Kenneth West, Mr Wayne Lonergan, and Professor Kenneth Lehn.
Background
3. From late 1982, Dr Robert Seamark and Dr Julian Wells (now deceased), research scientists at the University of Adelaide, worked on a collaborative project to develop transgenic technology with the objective of producing more food efficient, faster growing and leaner pigs. The University's intellectual property in this and other research was vested in Luminis Pty Ltd (``Luminis''), which formed a start up company that subsequently, in 1987, became an unlisted public company limited by shares, Bresatec Ltd (``Bresatec''). On 29 February 1988, Bresatec purchased the intellectual property in the transgenic technology from Luminis in consideration of the issue to Luminis of four million $1 shares. The
ATC 2131
valuation of the technology in Bresatec's accounts (Exhibit A31, p 18) is a historical reflection of this purchase.4. On 29 June 1989, Bresatec concluded an agreement with Hambro-Grantham Ltd (``Hambro-Grantham'') and Cambooya Pty Ltd (``Cambooya''), a company associated with John P Fairfax Pty Ltd, whereby each company purchased approximately 20% of Bresatec shares for a consideration of $1M. On 1 April 1991, Bresatec concluded an agreement with American Cyanamid Company (``Cyanamid'') whereby Bresatec, in consideration of a payment of $250,000, granted Cyanamid two options exercisable within 12 months: first, an option to purchase a licence for Bresatec owned technology and, second, an option to purchase shares in Bresatec. If the options were exercised, the consideration of $250,000 was also convertible into shares. In March 1992, Cyanamid exercised the second option with the result that it invested $2.25M in Bresatec shares, and obtained an extension to the first option to purchase a licence in respect of Bresatec technology until 28 February 1998.
5. In the latter part of 1991, Dr John Smeaton, the Managing Director of Bresatec, commenced discussions with Mr Daniel Phillips of MBL with a view to the formation of a joint venture Syndicate between Bresatec and MBL to provide research and development (``R and D'') funding for Bresatec. Under the terms of the provisions of the Income Tax Assessment Act 1936 (``the Act''), MBL could obtain various tax deductions in respect of its expenditure in relation to the syndicate provided the R and D was approved by the Industry Research and Development Board (``IRDB''), and it could also obtain an advance opinion on such a syndicate from the Respondent by making a Tax Ruling Request.
6. In March 1992, MBL engaged a valuer, Dr Maurice Venning, to undertake an independent evaluation of the project and a valuation of the core technology. On 16 June 1992, the IRDB determined that the proposed project satisfied the definition of ``research and development'' under s 73B(1) of the Act. On 25 June 1992, Dr Venning sent his final report to Mr Phillips and Dr Smeaton stating that he considered a value of $15.35M for the core technology used by the Syndicate to be a reasonable one.
7. On 30 June 1992 and 1 July 1992, a number of transaction agreements were concluded as a result of which the following arrangements were made:
- (a) Bresatec granted Luminis a 15 year non- exclusive licence in respect of the Bresatec owned transgenic pig technology in consideration of a payment of $269,000 and a royalty. On 16 September 1992, an amended agreement was concluded whereby the licence granted was exclusive.
- (b) MS3 and Bresatec Investments Pty Ltd (``Bresatec Investments'') entered into a joint venture syndicate (``the Syndicate'') in the ratio respectively of 99:1. MS3's equity in the Syndicate amounted to $27,645,750 and Bresatec Investments' amounted to $279,250. MBL was appointed to manage the Syndicate, incurring management fees of $425,000 (including set up costs of $150,000) to MBL.
- (c) MS3's equitable investment in the Syndicate of $27,645,750 comprised equity capital of $16,755,750 and debt funding of $10,890,000 borrowed from Macquarie Acceptances Ltd (``MAL''), another subsidiary company of MBL.
- (d) Bresatec Investments' equitable investment in the Syndicate of $279,250 comprised equity capital of $169,250 and debt funding of $110,000 from its parent company Bresatec.
ATC 2132
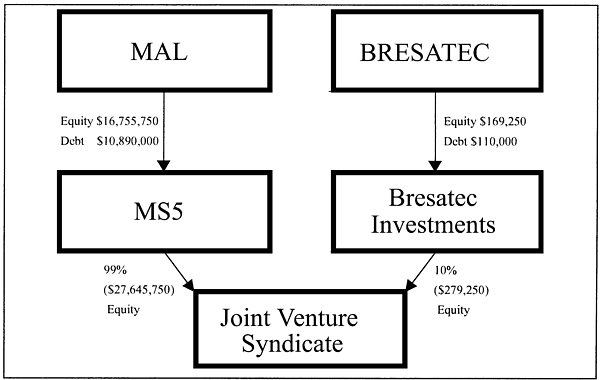
- (e) Luminis granted the Syndicate a 15 year exclusive licence/sub-licence in respect of Luminis' embryo stem cell (``ESC'') technology and Bresatec's transgenic pig technology (``the core technology'') in consideration of $15,350,000 (``the core technology licence fee''). (This was attributable in the relative proportions $15,196,500 to MS3 and $153,500 to Bresatec Investments.)
- (f) The Syndicate appointed Luminis to carry out a program of R and D in consideration of $12,150,000 comprising a $9,000,000 R and D budget plus an R and D profit margin of $3,150,000. The agreements provided for an R and D period of three years followed by a commercialisation period of five years. Luminis, being wholly owned by the University of Adelaide, had a tax- exempt status. Luminis deposited $27,225,000 with Macquarie Finance Ltd (``Macquarie Finance''), from which it drew down $8,910,000 in four instalments to pay for the conduct of the R and D which it had sub-contracted to Bresatec.
- (g) The Syndicate granted a marketing option to Bresatec Marketing Pty Ltd (``Bresatec Marketing'') which, if exercised, entitled Bresatec Marketing to market products resulting from the commercialisation of the Syndicate's core technology in consideration of the payment of a licence fee of 15% of gross commercialisation income derived prior to completion of the R and D programme and, thereafter, 20% of the gross income actually received or receivable during the marketing period (D5, p 9).
- (h) Bresatec granted to MAL a put option exercisable at the conclusion of the commercialisation period in 2000 whereby Bresatec would be required to purchase from MAL the issued share capital in MS3 for a price equal to the put option price, as at the put option settlement date. The agreement was later amended, in October 1992, to the effect that ``the Put Option Price is $45,350,817.06'', payable on the review date (CT103; D19).
8. The transaction agreements were conditional upon a satisfactory ruling by the Respondent in respect of the Syndicate. A request for a ruling was made on 14 July 1992 (C132) and an advance opinion was provided on 27 August 1992 (C134).
9. In its income tax return for the substituted financial year ended 30 September 1992, MS3 claimed an allowable deduction of $15,196,500
ATC 2133
in respect of its 99% share of the core technology licence fee, a deduction of $5,060,085 for its share of R and D costs and management fees, and a deduction in respect of interest of $323,999. As a result of the allowable deductions, MS3 made a loss for that year of $20,580,584 and also losses in subsequent financial years, which were transferred to other wholly owned subsidiaries of MBL (CT136).10. On or about 15 June 1993, MS3 transferred to the Applicant a loss of $109,947. During its substituted financial years ending 30 September 1992 to 30 September 2001 (inclusive), MS3 transferred losses in the same way to 13 other wholly owned MBL subsidiaries (CT136, p 1253).
11. Bresatec concluded the projected R and D on 30 June 1995, with all the R and D funds being spent. Over the three year R and D period, Bresatec produced several hundred transgenic pigs. During this period, quarterly technical and investor reports were produced. Investor and Technical Review meetings were also held on a quarterly basis and were attended by members of the Syndicate committee, including Mr Phillips, Mr Goode, Dr Venning, Dr Smeaton, Dr Robins and Mr Hart. These meetings reviewed the progress of the Syndication including the progress of the transgenesis program, the ESC technology, and the staffing of the project. During the R and D period, attempts were also made to commercialise the core technology, with discussions being held with pig producers in Australia, the US and Europe.
12. At the same time, approaches were made to the Australian regulatory authorities for approval of the sale of transgenic pigs for human consumption. When discussions with the authorities failed to produce any regulatory approval, and in the absence of any relevant standards or guidelines, in about February 1996, the Board of Bresatec decided not to proceed with the sale of transgenic pigs because of concerns about legal liability. Soon after, Bresatec slaughtered and buried about 300 transgenic pigs, semen from the best transgenic boars was frozen, and the project was put on hold.
13. In December 1995, Bresatec changed its name to BresaGen Ltd (``BresaGen''). Cyanamid's option to purchase a licence in respect of Bresatec technology expired on 28 February 1998. In September 1999, BresaGen was floated on the Australian Stock Exchange with ``a market capitalisation as high as $90M'' (Smeaton, A8, p 79). In 1999, the Food Acts were modified to allow the sale of genetically manipulated organisms. In 2001, BresaGen signed an agreement with an international pig company to continue its research into transgenesis with a view to commercialising transgenic pigs.
14. On 30 September 2000, MAL exercised its put option and BresaGen purchased all MAL's shares in MS3 and the debt of $28,449,330 (comprising a debt of $10,890,000 plus capitalised interest of $17,559,330 in respect of the period 1992 to 2000) for $45,380,817. This was covered by the deposit in Luminis' name, accruing since 1992, with Macquarie Finance.
15. On 25 August 2000, the Respondent issued two determinations to MS3 for the financial year ending 30 September 1992:
- (1) the deduction for the core technology licence fee was reduced from $15,196,500 to nil; and
- (2) the deduction for interest expenditure was reduced from $323,999 to $145,901.
16. On 31 August 2000, the Respondent issued an adjustment sheet to the Applicant reducing the deduction claimed by the Applicant for the loss transferred from MS3 from $109,947 to nil. On or about 31 August 2000, the Respondent issued an adjustment sheet to MS3 adjusting to nil MS3's net loss for the financial year ending 30 September 1992.
17. During September and October 2000, the Respondent issued Assessments to MS3, MBL and a number of its subsidiary companies including the Applicant. The Assessment issued to the Applicant on 11 October 2000 increased its taxable income for the financial year ending 30 September 1992 by $109,947. On 14 November 2000, the Applicant lodged an Objection against the Assessment. On 26 July 2001, the Respondent disallowed that Objection. On 20 September 2001, the Applicant lodged an application with the Tribunal for a review of that decision.
18. In total, 18 applications for review by MBL and its subsidiaries were lodged with the Tribunal. In correspondence between the parties in November/December 2001, it was agreed that the application by the Applicant would be
ATC 2134
treated as representative of the issues arising in relation to the transfer of losses and their deductibility for all the relevant MBL group companies.Applicable legislation
19. The relevant legislation is the Income Tax Assessment Act 1936. The key issues to be determined by the Tribunal and the relevant legislation are as follows:
- (1) Was MS3 entitled to an allowable deduction of $15,196,000 in the financial year to 30 September 1992 pursuant to s 73B(12)?
Section 73B(12) states:
``Subject to this section, where an eligible company incurs core technology expenditure during a year of income, the amount of that expenditure is allowable as a deduction from the assessable income of the company of the year of income.''
The following definitions appear in s 73B:
``73B(1AB) [`Core technology'] For the purposes of this section, technology is core technology in relation to particular research and development activities if:
- (a) the purpose of the activities was or is:
- (i) to obtain new knowledge based on that technology; or
- (ii) to create new or improved materials, products, devices, processes, techniques or services to be based on that technology; or
- (b) the activities were or are an extension, continuation, development or completion of the activities that produced that technology.
73B(1) ...
`core technology expenditure' , in relation to an eligible company, means expenditure incurred by the company after 7 September 1989 in acquiring, or in acquiring the right to use, technology for the purposes of research and development activities carried on by or on behalf of the company, being technology that is core technology in relation to those activities;''
- (2) Was MS3 entitled to an allowable deduction of $178,098 in respect of interest paid by MS3 to MAL in the subject year of income?
- Section 73B(14) permits a deduction for a company's assessable income in respect of R and D expenditure where the aggregate amount is greater than $20,000.
- Section 73B(34) provides that if the IRDB gives to the Respondent a certificate stating whether particular activities carried out by an eligible company were research and development activities as defined in s 73B(1), that certificate is binding on the Respondent for the purpose of making an assessment of the company's taxable income.
- Section 73CA provides for a deduction of 100% where the expenditure of research and development funds is determined by the Commissioner as not at risk in respect of the expenditure.
20. In determining these two issues, reference must be made to the relevant anti-avoidance provisions in the Act relied on by the Respondent in making his determination.
First, section 73B(31) states:
``Where-
- (a) an eligible company has incurred an amount of research and development expenditure, an amount of core technology expenditure, or an amount of expenditure in the acquisition or construction of plant, a building or an extension, alteration or improvement to a building for use by the company exclusively for the purpose of the carrying on by or on behalf of the company of research development activities; and
- (b) the Commissioner is satisfied that-
- (i) having regard to any connection between the company and the person to whom the expenditure was incurred and to any other relevant circumstances, the company and that other person were not dealing with each other at arm's length in relation to the incurring of that expenditure; and
- (ii) the amount of that expenditure would have been less if the company and that other person had dealt with each other at arm's length in relation to the incurring of that expenditure,
ATC 2135
so much only of that expenditure as the Commissioner considers reasonable having regard to-
- (c) the connection between the company and that other person;
- (d) the amount of the expenditure that would, in the opinion of the Commissioner, have been incurred by the company if the company and that other person had dealt with each other at arm's length in relation to the incurring of that expenditure; and
- (e) such other matters as the Commissioner consider relevant,
shall be taken into account for the purpose of this section.''
21. The relevant questions to which this subsection gives rise are:
- (a) having regard to any connection between the Syndicate and Luminis and to any other relevant circumstances, is the Respondent (in these proceedings the Tribunal) satisfied that the Syndicate and Luminis ``were not dealing with each other at arm's length in relation to the incurring of that expenditure''?
If the Respondent (the Tribunal) is satisfied that the Syndicate and Luminis were not dealing at arm's length in relation to that expenditure, then the second question to which the subsection gives rise is:
- (b) whether the Respondent (the Tribunal) is satisfied that the amount of that expenditure would have been less had they been dealing with each other at arm's length in relation to the incurring of that expenditure?
If the Respondent (the Tribunal) is so satisfied, then the Respondent (the Tribunal) must determine how much of that expenditure should reasonably be taken into account having regard to the matters referred to in subparagraphs (c), (d) and (e).
22. The second anti-avoidance provision relied on by the Respondent is contained in Part IV A of the Act. Section 177D states:
``This Part applies to any scheme that has been or is entered into after 27 May 1981, and to any scheme that has been or is carried out or commenced to be carried out after that date (other than a scheme that was entered into on or before that date), whether the scheme has been or is entered into or carried out in Australia or outside Australia or partly in Australia and partly outside Australia, where-
- (a) a taxpayer (in this section referred to as the `relevant taxpayer' ) has obtained, or would but for section 177F obtain, a tax benefit in connection with the scheme; and
- (b) having regard to-
- (i) the manner in which the scheme was entered into or carried out;
- (ii) the form and substance of the scheme;
- (iii) the time at which the scheme was entered into and the length of the period during which the scheme was carried out;
- (iv) the result in relation to the operation of this Act that, but for this Part, would be achieved by the scheme;
- (v) any change in the financial position of the relevant taxpayer that has resulted, will result, or may reasonably be expected to result, from the scheme;
- (vi) any change in the financial position of any person who has, or has had, any connection (whether of a business, family or other nature) with the relevant taxpayer, being a change that has resulted, will result or may reasonably be expected to result, from the scheme;
- (vii) any other consequence for the relevant taxpayer, or for any person referred to in subparagraph (vi), of the scheme having been entered into or carried out; and
- (viii) the nature of any connection (whether of a business, family or other nature) between the relevant taxpayer and any person referred to in subparagraph (vi),
it would be concluded that the person, or one of the persons, who entered into or carried out the scheme or any part of the scheme did so for the purpose of enabling the relevant taxpayer to obtain a tax benefit in connection with the scheme or of enabling the relevant taxpayer and
ATC 2136
another taxpayer or other taxpayers each to obtain a tax benefit in connection with the scheme (whether or not that person who entered into or carried out the scheme or any part of the scheme is the relevant taxpayer or is the other taxpayer or one of the other taxpayers).''
23. ``Scheme'' is defined in s 177A(1):
```Scheme' means:
- (a) any agreement, arrangement, understanding, promise or undertaking, whether express or implied and whether or not enforceable, or intended to be enforceable, by legal proceedings; and
- (b) any scheme, plan, proposal, action course of action or course of conduct;''
24. Section 177A(5) states:
``A reference in this Part to a scheme or part of a scheme being entered into or carried out by a person for a particular purpose shall be read as including a reference to the scheme or part of the scheme being entered into or carried out by the person for 2 or more purposes of which that particular purpose is the dominant purpose.''
25. The relevant questions to which these provisions give rise are first, was there a ``scheme'', secondly, did the relevant taxpayer gain a ``tax benefit'' in connection with the scheme and, thirdly, did the taxpayer do so for the purpose of obtaining a tax benefit?
26. ``Tax benefit'' is defined in s 177C so as to include:
- ``(b) a deduction being allowable to the taxpayer in relation to a year of income where the whole or part of that deduction would not have been allowable, or might reasonably be expected not to have been allowable, to the taxpayer in relation to that year of income if the scheme had not been entered into or carried out;''
27. The Tribunal notes that the relevant provision in relation to deductions for losses and outgoings is s 51(1):
``51(1) All losses and outgoings to the extent to which they are incurred in gaining or producing the assessable income, or are necessarily incurred in carrying on a business for the purpose of gaining or producing such income, shall be allowable deductions except to the extent to which they are losses or outgoings of capital, or of a capital, private or domestic nature, or are incurred in relation to the gaining or production of exempt income.''
Evidence as to the facts
Dr Robert Seamark
28. Dr Seamark is Director of the Flinders Medical Research Institute and Chair of its Advisory Board. He was employed by the University of Adelaide from 1965 to 1996, from 1969 in the Department of Obstetrics and Gynaecology. In his statement (A4), he said that in late 1982/early 1983, he commenced a collaborative project with Dr Julian Wells of the Department of Biochemistry to develop transgenic technology of direct interest to the livestock industry. Dr Smeaton's initial aim was to create a new breed of pig with increased growth efficiency, by incorporating extra copies of controllable pig growth hormone genes into the pig's genome. He speculated that this technology had the potential to revolutionise the pig production market and could be adapted to other livestock to enhance their production characteristics.
29. Between 1982 and 1985, Dr Wells and his colleagues in the Department of Biochemistry developed gene constructs, comprising sequences of DNA, to control the expression of pig growth hormones. Under Dr Seamark's supervision, these constructs were inserted into pig cells in the laboratory to create a growth hormone gene triggered by certain concentrations of metals in the pig's body, for example, zinc or copper administered through dietary supplementation. In 1986, six transgenic pigs were produced after microinjection of the porcine growth hormone fusion gene into the nucleus of fertilised eggs, one of whom evidenced enhanced food conversion, reaching market weight sooner than conventional pigs without any adverse effect on the pig's health. In April 1987, a patent application was lodged in Australia to protect the transgenic technology developed and, in 1988, Dr Seamark, Dr Wells and others published an article on this work in the Journal of Cell Science.
30. At that time, the research team was experiencing various limitations with the transgenic technology which required further R and D. However, by 1992, the team had clear evidence of the inheritance of the ``transgene'' in the next generation of pigs following natural
ATC 2137
mating, and that the transgene could be switched on and off by modifying the animal's diet. While success to date had largely been in the laboratory, the transition to commercialisation had begun.31. During this period, the team was also researching the use of ESC technology as a means of improving the growth characteristics of livestock. The ESC technology developed was considered to be more efficient and reliable and was thought to have greater potential in achieving the objective of an improved growth rate for livestock animals. In the early 1990s, Dr Seamark and Dr Wells were also considering how the use of their transgenic technology might lead to collaborations allowing the development of a xenotransplantation capacity in Australia. Xenotransplantion involves the transplantation of the cells, tissues or organs from one species of animal to another.
32. In 1991, Dr Seamark became aware that Bresatec was negotiating with MBL with a view to entering into an R and D syndicate to raise funds for a three year combined transgenesis and ESC research program. Dr Seamark believed that funds of $9M would enable the research team to overcome current problems with the transgenic technology and found a commercially valuable breeding stock. It would also advance the research team's understanding of ESC technology and allow them to apply this in advancing the production characteristics of the pig, and to facilitate better understanding of the use of this technology in such applications as xenotransplantation and cloning.
33. Dr Seamark agreed that it was likely that the commercial product of this research might be controversial. There were groups who, for religious or other strongly held reasons, said that interfering with nature was wrong, and the federal and State regulators were not prepared to give unequivocal approval. Although Dr Seamark was confident that such issues could be addressed in time, he acknowledged that he is not aware of any transgenic pig meat or other animal flesh being sold in Australia today. Nevertheless, he said transgenic pig meat is absolutely safe. He described his eating of this being televised and of barbecuing the meat in the Rundle Mall in Adelaide. He said there was general acclaim because the meat is lean and because the skin is slightly thickened and produces very nice crackling!
34. Dr Seamark resigned from the University of Adeliade in 1995 to take up a position in Canberra and has had no further involvement with the Syndicate since that time.
Dr Allan Robins
35. Dr Robins is Senior Vice President and Chief Scientific Officer of BresaGen Inc, a wholly owned subsidiary of BresaGen Ltd. He was employed by Bresatec from 1991, and previously, since 1982, in the Department of Biochemistry of the University of Adelaide. He was a PhD student in Dr Wells' laboratory in the Department from 1976 and, over many years, worked with Dr Seamark, Dr Wells and others on various aspects of gene and reproductive technology.
36. In his statement (A5), Dr Robins said that as at 1991/1992, the University of Adelaide team had the most advanced transgenic pig research project in the world, something which was confirmed by American Cyanamid's investment in Bresatec in 1991. Nevertheless, the team were still experiencing technical and commercial problems with the transgenic technology. In particular, the technology had yet to be tested in a large-scale commercial environment. While Dr Robins agreed that, in 1992, millions of dollars were still required in research funding, he said that, nevertheless, he thought that field trials could be completed within three years.
37. Dr Robins said field trials to breed transgenic animals and test their progeny were completed in 1995 or 1996 but, at that time, it became obvious that there would be regulatory requirements that they had not anticipated. Dr Robins said he had been involved in earlier discussions with South Australian regulators who had then been very positive about regulatory approval. In 1992, they could not have predicted that genetically modified food was likely to be a huge consumer issue. Dr Robins said the project team had thought they would have a marketing advantage because their product was leaner and a better quality product as a result of a genetic modification that was entirely safe. However, in 1995/1996, the pig producers, Bunge Meat Industry, with whom they were working, were unwilling to conduct further field trials because they were concerned about a consumer backlash. As a result, the project has since been on hold.
ATC 2138
38. Dr Robins was asked about Monsanto and the use of bovine somatotropin (``BST'') to increase milk production in dairy cows. He said BST poses no risk to human consumers. He distinguished Monsanto's use of BST from Bresatec's transgenic pig project because while the former sought to increase the quantity of the milk by injecting cows with BST, the latter sought to improve the quality of the product by genetic modification and selective breeding. Nevertheless, Dr Robins acknowledged that he was not aware of any transgenic animal flesh being sold in the US.
Dr John Smeaton
39. Dr Smeaton is President and Chief Executive Officer of BresaGen. He was formerly a Director and the Managing Director/ Chief Executive Officer of Bresatec from late 1987.
40. In his statement (A8), Dr Smeaton said one of his main responsibilities has been to seek and obtain funds to finance ongoing research. Until the stock market crash in October 1987, it had been intended to float Bresatec soon after 1987. After the stock market crash, this was postponed and the Bresatec Board decided to look to private investors for funds for further research. Ultimately, BresaGen was listed on the Australian Stock Exchange in September 1999.
41. During 1987, Dr Smeaton had discussions with Metro Farms Pty Ltd and its associated company Metro Meat (Holdings) Pty Ltd (``Metro Meat''), part of the Adsteam Group of companies, about the possibility of Bresatec and Metro Meat entering into a joint venture to develop and exploit the transgenic technology and protein production technology in pig production. An agreement between Bresatec and Metro Meat was signed on 23 March 1988. Between 1988 and 1990, Metro Meat paid approximately $2M into the joint venture for R and D that was mostly undertaken at Metro Meat's piggeries in South Australia. On 12 March 1991, Bresatec purchased Metro Meat's share in the joint venture when the Adsteam Group was experiencing financial difficulties.
42. In about June 1988, Dr Smeaton commenced discussions with Hambro- Grantham and Cambooya, about a possible investment in Bresatec. This culminated in an agreement dated 29 June 1989 whereby they each invested $1M in Bresatec in consideration of approximately 20% of Bresatec's shares. As at December 1989, the approximate shareholdings in Bresatec comprised Luminis 60%, Hambro-Grantham 19%, Cambooya 19%, and employee shareholders 2% (A8, p 12).
43. From 1988, Dr Smeaton also commenced discussions with various overseas companies with a view to an alliance, including from 1990 with Mr Robert Mooney of Cyanamid. On 1 April 1991, Bresatec entered into an agreement with Cyanamid whereby:
- (a) Bresatec granted Cyanamid an option to purchase the licence of various Bresatec technology;
- (b) Bresatec granted Cyanamid an option to purchase 1,282,051 new ordinary shares at $1.56 per share for a total of $2M (A48);
- (iii) in consideration of the grant of these options, Cyanamid paid Bresatec $250,000, convertible to an additional 160,257 Bresatec ordinary shares on the exercise of the options.
Cynamid exercised the option to purchase shares in November/December 1991.
44. Dr Smeaton stated that in mid 1991, Bresatec had produced a number of healthy transgenic pigs by insertion of the porcine growth hormone GH gene, and had achieved ``some level of control of expression in the transgenes in mice''. In the pig, control was sought of the transgene's expression of a protein (a growth hormone), naturally occurring in the pig, to promote the more efficient growth of the pig and the production of a better quality animal.
45. Dr Smeaton said that in 1988, Bresatec had regulatory approval from the National Health and Medical Research Council (``NH & MRC'') and the Food Technology Council to sell transgenic pigs, and a few pigs were sold into the food chain at about that time. Dr Smeaton said that in May/June 1992, he did not anticipate there would be regulatory problems with the technology because there was no conceivable risk to human health. However, in 1995, the regulatory environment was more uncertain and Bresatec decided against the risk of selling transgenic meat in the market place in the absence of further assurance of regulatory approval, even though the transgenic meat was completely safe for human consumption.
46. Dr Smeaton said it was about July 1991 that he first had a discussion about Bresatec
ATC 2139
raising research funds through an R and D syndication and received an ``indicative proposal'' from MBL. Dr Smeaton could not recall mentioning, in these early discussions, the need for between $5M and $9M in research funds. He said his preferred figure of $9M would have been based on Bresatec estimates. While no valuation of the transgenic technology was undertaken for the agreement with Cyanamid, Dr Smeaton considered $15M to $25M was a fair value, and, with hindsight, thinks this was probably conservative. He based his view on his understanding of the technology and on the commercial information he gained from his discussions with other companies during his travels on behalf of Bresatec both in Australia and overseas. Dr Smeaton said Bresatec would not have been able to raise the additional research funds from its existing shareholders. They were aware of the MBL Syndicate proposal and supported ongoing negotiations towards the conclusion of an appropriate agreement. Cynamid considered a successful syndication to be important for their ongoing relationship with Bresatec.47. Dr Smeaton said he first met Mr Daniel Phillips of MBL in early December 1991, when he told Mr Phillips his view of the value of Bresatec's core technology and what was required in research funding. He also told him of the Cyanamid transaction, which valued Bresatec at about $12M. Mr Phillips said he would need an independent valuation.
48. After this meeting, Mr Phillips sent Dr Smeaton a modified indicative proposal assuming a value of $12.2M for the core technology. This formed the basis of further discussions, but it was not until February 1992 that Dr Smeaton realised that Brescatec would be liable to pay tax on the core technology licence fee. At a Brescatec Board meeting on 19 February 1992, it was agreed that Luminis should be included in the proposed Syndicate structure to gain the benefit of its tax-exempt status. Luminis would, in any event, have had some involvement because it owned some of the relevant intellectual property, in particular in relation to the ESC technology, which it had always been intended would be part of the core technology included in the Syndicate transaction.
49. By fax dated 26 February 1992, MBL advised Dr Smeaton of the names of two valuers who would contact him to give him a quotation for a valuation of the core technology licence. One of these, Dr Maurice Venning, met with Dr Smeaton and Dr Robins in Adelaide on 17 March 1992. Dr Smeaton acknowledged that one of the purposes of the meeting was to see whether it was likely that Dr Venning's valuation would come within the same ``ballpark'' as Dr Smeaton's, which would determine whether it was worth going ahead with the proposed Syndicate. Following the meeting, Dr Smeaton formed the view that Dr Venning had a strong grasp of the science and technology involved and would take an independent view in making the valuation. However, because Dr Venning seemed to be impressed by Bresatec's research, Dr Smeaton was optimistic that Dr Venning's figure would probably be within the same ballpark. Following this meeting, Dr Venning was formally instructed to conduct a valuation by MBL although it was agreed that Bresatec would be responsible for paying his fees.
50. Dr Smeaton acknowledged that he asked Dr Venning to comment on Dr Smeaton's draft letter to the IRDB and that Dr Smeaton had included a paragraph suggested by Dr Venning, stating that the project represented ``an attractive opportunity for prospective research and development investors to invest in a totally indigenous technology with commercial potential''. He did not consider it was inappropriate to seek assistance from the independent valuer. He said Dr Venning would have told him that he had some experience or expertise in the area and offered to look over the draft. Dr Smeaton said he considered this a totally separate matter to the valuation.
51. Dr Smeaton was asked about his ongoing contact with Dr Venning in about May/June 1992. He acknowledged that he told Dr Venning on two or three occasions that he thought the core technology was worth $15M to $25M, but Dr Venning made it clear that he would determine the valuation independently. Dr Smeaton said he never discussed with Dr Venning the methodology Dr Venning was using for his valuation.
52. Dr Smeaton said, at about this time, he became concerned that if there was a change in the tax rate, Bresatec might have to ``top up'' the funds available to pay MBL if they chose to exercise the put option. Neither he nor the Bresatec Board wanted Bresatec to bear this risk.
ATC 2140
53. Dr Smeaton was asked about the licence fee of $269,000 paid by Luminis to Bresatec for use of the core technology and the $279,000 invested by Bresatec in the Syndicate, equivalent to 1%. Dr Smeaton said the parties involved in negotiating this agreement were Dr Richard Gregson of Hambro-Grantham, Chair of the Besatec Board's subcommittee considering the MBL proposal, Mr Hart, the Managing Director of Luminis, and himself.
54. Dr Smeaton was asked how the Respondent's decision is affecting BresaGen currently. He said the current proceedings are adversely affecting market sentiment and are having a downward effect on BresaGen's share price. There have been rumours on the market that Bresatec's funds are at risk. He has made a limited disclosure in BresaGen's annual report, stating that there is a tax issue.
55. Dr Smeaton said his primary purpose in having Bresatec enter into the Syndicate was to obtain funding for advancing Bresatec's research to a point at which it could be commercialised successfully. Ultimately, Bresatec's aim was to be listed on the Stock Exchange and to become a major local and international biotechnology company. He said that all of the $9M in research funds advanced by the Syndicate was expended in the projected research and was the subject of quarterly technical and investor reports. Over the research period, Bresatec produced several hundred, prototype stage, transgenic pigs. However, when discussions with the NH & MRC failed to produce regulatory approval for the sale of transgenic pigs for human consumption, and in the absence of any relevant standards or guidelines, in about February 1996, the Bresatec Board decided not to proceed with the sale of its transgenic pigs because of concerns about legal liability. Soon after, Bresatec slaughtered and buried about 300 transgenic pigs. Semen from the best transgenic boars was frozen and the project was put on hold.
56. Dr Smeaton stated that in 1999, the Food Acts were modified to allow the sale of genetically manipulated organisms (``GMOs''). While the sale of transgenic pigs is now outside BresaGen's core business, in 2001 BresaGen signed an agreement with an international pig company to sponsor BresaGen's continuing research into transgenesis with a view to commercialising transgenic pigs.
57. In his statement, Dr Smeaton concluded that the major stumbling block to the success of the Syndicate was the failure of the Federal, State and Territory Governments to introduce regulations governing the sale of GMOs and, in particular, transgenic pigs. Nevertheless, the technology developed during the life of the Syndicate assisted Bresatec in other ways, under-pinning its work on protein drugs and ESC based products, which enabled BresaGen to proceed with its stock market floatation in September 1999 and to establish its reputation as a leading biotechnology company.
Mr Peter Hart
58. From January 1990 until February 2000, Mr Hart was Managing Director of Luminis, which was the Trustee of the Luminis Investment Trust, the primary beneficiary of which was University of Adelaide. In his statement (A7), Mr Hart said from 1990 to 2000, Luminis' principal activities included the commercialisation of the University's intellectual property and the establishment and management of contract, research and consultancy work for University staff. From 1990 to 1996, Mr Hart was an alternate director of Bresatec and, from September 1996, a director of BresaGen.
59. Mr Hart said that in 1990 he considered Bresatec the most successful of the University's start up companies. However, while Luminis provided intellectual property to Bresatec for the development of its business, its role was not to provide research funding.
60. Mr Hart became familiar with R and D syndicates through a proposal put to him by Bankers Trust in 1990 that did not, ultimately, go ahead. No investors were interested in the proposed syndicate. Then in July 1991, Dr Smeaton informed Mr Hart that a proposal had been made by MBL. Mr Hart recalled discussion of MBL's ``indicative proposal'' at a Bresatec Board meeting. At first, Mr Hart did not consider that Luminis or the University's intellectual property would be involved but, by December 1991, he realised that it would be necessary for Luminis to be involved in the proposal. He was asked what he knew of the figures discussed in relation to the proposed arrangement. He said his primary interest was in relation to the research funding that the figures would generate for Bresatec. He believed that what was being proposed was a commercial relationship. He did not recall the
ATC 2141
origin of the first proposed core technology value of $12.2M but said he thought this figure was needed to generate research funds of $9M. Similarly, if the core technology was valued at $8.2M, only $6M in research funding would be generated. He was aware from his discussions with University researchers that the technology was valuable, but has no recollection of a specific value being mentioned.61. Mr Hart became aware that a tax ruling was likely to be a major issue. Luminis became part of the Syndicate instead of Bresatec because of Luminis' tax-exempt status: to avoid the tax consequences that would have followed if Bresatec had received the core technology licence fee. However, while Luminis would have had to be involved in any event because it owned the intellectual property in the ESC technology - in value worth twice as much as Bresatec's intellectual property, Mr Hart did not consider it appropriate for Luminis to become an equity investor in the Syndicate.
62. Mr Hart was asked about the $15.35M valuation of the core technology. He was aware that if the valuation was less than this, research funding of $9M would not be achieved. He also acknowledged that a valuation of $15.35M achieved a shift of the risk flowing from a change in the tax rate from Bresatec to MBL.
63. Mr Hart agreed that part of the Syndicate transaction involved Luminis paying a licence fee of $269,000 to Bresatec as part of the consideration for the use of Bresatec's intellectual property for a period of 15 years. He acknowledged that this figure was not arrived at by arm's length negotiation. It was a figure which was inserted in the documents that did not reflect the true value of the Bresatec core technology. Mr Hart said he did not know who nominated this figure. It was not important to Luminis because it did not form part of the benefit flowing from the transaction. He agreed there was some relationship between the licence fee of $269,000 paid to Bresatec and the $279,000 paid by Bresatec as its contribution to the Syndicate. Both sums were part of the total financial picture. Mr Hart could not recall whether Luminis paid Bresatec's legal fees associated with the agreement amounting to about $6,000.
64. Mr Hart's attention was drawn to the request by MBL for a tax ruling in relation to the Syndicate. The request includes reference to various limbs of the Transgenic Participation Agreement including the licence granted to Luminis by Bresatec to use Bresatec's core technology, and states that the Agreement was a result of an arm's length negotiation between Bresatec and Luminis. Mr Hart said there was a significant negotiation between Dr Smeaton and himself as to some of the terms and conditions in the agreement prior to its final signature. Mr Hart could not, however, be any more specific than this.
Mr Daniel Phillips
65. In his statement (A9), Mr Phillips stated he is the Executive Director of the Investment Bank Group division of MBL, a position he has held since July 1996. Previously, he was employed in the Corporate Finance Division. Early in 1991, this division assumed responsibility for MBL's involvement with other companies in R and D investments for MBL. He therefore sought to spread MBL's investments across a portfolio of diverse, high quality and innovative R and D projects. He understood that the Federal Government wished to encourage syndicated R and D as a means of attracting private capital for innovative projects.
66. Mr Phillips included a put option in MBL's R and D investments so that the investments were not at risk and MBL could exit a transaction. If the research was commercially successful so that MBL received royalty payments, MBL could choose not to exercise the put option and remain in the Syndicate. In selecting appropriate projects, Mr Philips looked at the reputation of the researchers and any business people already involved in the project. He relied on the IRDB's review of the technology and proposed commercialisation and on an advance opinion from the Respondent and the ATO's confirmation that a proposed syndicate was satisfactory. He also relied on an independent valuer for an evaluation of the proposed core technology and research, and for a market valuation of the core technology. His rule of thumb was that the R and D funding should be 60%-70% of the value of the core technology. In 1991/1992, Mr Phillips was involved in six proposed R and D syndicate arrangements.
67. Mr Phillips' first involvement with Bresatec was in November 1991 when he spoke on the telephone with Dr Smeaton to follow up an indicative proposal for a research syndicate which had been sent to Dr Gregson in July/ August 1991. Dr Smeaton indicated that
ATC 2142
Bresatec was seeking research funds of between $5M and $9M. Following this, on 12 December 1991, Mr Phillips and Mr David Goode, a financial analyst at MBL who was assisting Mr Phillips, met with Dr Smeaton in Adelaide and, later that day, at the University of Adelaide, met with Dr Wells and Dr Seamark. Mr Phillips formed the view that the proposed R and D had significant potential and he was impressed by the involvement of Cyanamid and the other corporate shareholders. Dr Smeaton told him that Bresatec was seeking around $9M in research funding over a period of three years. Mr Phillips assumed the core technology was worth about $12.2M. He was comfortable with this because Dr Smeaton told him that between $6M and $7M had already been spent on developing the technology and because the Cyanamid investment had valued Bresatec at approximately $12M.68. Mr Phillips said that Dr Venning was appointed by MBL to undertake the evaluation of the project and value the core technology. Dr Venning had previously been engaged by MBL to prepare a valuation in relation to another R and D syndicate. Mr Phillips had been impressed by his technical knowledge, his commercial approach and because he had been protective of his independence. Mr Phillips phoned Dr Venning and asked him to phone Dr Smeaton about undertaking an independent valuation. In a letter to Dr Venning dated 14 April 1992 (C52), Mr Phillips set out the matters to which he should have regard in his valuation. If Mr Phillips had thought Dr Venning's report was not independent he would have rejected it.
69. Mr Phillips was aware of ongoing contact between Dr Venning and Dr Smeaton, which he assumed was for the purpose of Dr Venning preparing his report. Mr Phillips did not consider the assistance that Dr Venning provided to Dr Smeaton in drafting a letter to the IRDB as a partisan act. In so doing, Dr Venning was assisting the Syndicate as a whole. Mr Phillips agreed that as he became more familiar with the proposed Syndicate, he became aware that regulatory approvals would be required. This was addressed in Dr Venning's report. Mr Phillips derived comfort from Cyanamid's involvement because regulatory approval would have been a matter they would have considered in making their investment.
70. Mr Phillips was asked about the term of the R and D and commercialisation periods. He said the Respondent was not concerned about the term of the R and D period - it was more a matter of how long it would take to complete the R and D. The five year commercialisation period had emerged in discussions with the Australian Taxation Office (``ATO'') as being a period they were comfortable with.
71. Mr Phillips was asked about MBL's spreadsheets. He said these indicated the consideration of a number of alternative scenarios based on different valuations for the core technology and projected yields for MBL of about 18%. Mr Phillips agreed that if there was a reduction in the company tax rate, MBL's tax deductions would have been reduced. Thus, the put option price was adjusted upwards to preserve MBL's position and maintain its yield, and the level of security required in the transaction also needed to be increased. However, at a meeting with the Bresatec Board, on 14 May 1992, it was suggested by Bresatec that the core technology was worth at least $15M and so the spreadsheets and proposal were adjusted accordingly. Mr Phillips could not recall whether he spoke to Dr Venning before making these adjustments. At the meeting, Mr Phillips agreed that MBL would bear the risk of a change in the tax rate. He acknowledged in evidence that MBL would benefit from an increased core technology fee by virtue of higher tax deductions.
72. With regard to the put option, if exercised by MBL, Bresatec would be required to purchase MS3's shares at a pre-determined price calculated on the basis of the core technology fee, the R and D funding, and interest. The deposit account in the name of Luminis in which the funds paid to Luminis by the Syndicate were invested, would be maintained so that it contained sufficient funds to meet the put option purchase price after the drawing down of the R and D funding.
73. Mr Phillips acknowledged that tax would ordinarily be payable by the recipient on the core technology licence fee paid by the Syndicate. In Bresatec's case, this potential shortfall was addressed by including Luminis, which was tax-exempt, as the licensor and recipient of the licence fee. This had no other effect on the Syndicate or the tax position of MBL. If the technology was commercialised successfully, Bresatec Marketing would receive
ATC 2143
the majority of the resultant income with payments to the Syndicate of 15% for the first three years and 20% for the next five years (D5).74. On 9 June 1992, Mr Phillips wrote to the IRDB enclosing an application for registration of the Syndicate. On 18 June 1992, he received Dr Venning's draft valuation of the core technology - of $10.4M to $13.5M in respect of the pig market application of the technology or $14.7M to $19M if medical applications of the technology were also taken into account. Dr Venning concluded a value of about $15M for the core technology used by the Syndicate was reasonable. After discussion with Mr Phillips on the phone and at his suggestion, Dr Venning subsequently amended the valuation to $15.35M, as being within his range of values (C114).
75. In April 1992, MBL established MS3 as a wholly owned subsidiary company with a view to entering into the Syndicate. The transaction documents were signed by the MBL parties on 1 July 1992. Mr Phillips said the Syndicate R and D concluded on 30 June 1995 as a technical success (A9, p 23). However, Bresatec ``got caught up in the controversy surrounding genetically modified foods'' and, despite significant effort and lobbying, failed to obtain regulatory approval. On 3 October 2000, MBL (through MAL) exercised its put option ``on the basis that in the circumstances that then prevailed, the Project was unlikely to be commercially successful'' (A9, p 24).
76. Mr Phillips was asked about a spreadsheet sent by MBL to Luminis on 12 October 1992 (CT104). This shows the put option price as approximately $45M and another figure of approximately $64M which, Mr Phillips said, indicated a scenario where, if MBL received royalties of approximately $64M, it would not be exercising its put option. He acknowledged that such an amount, bearing in mind that Bresatec Marketing was entitled to keep 85% of the revenue in the first three years and 80% thereafter, meant that the total revenue generated by Bresatec Marketing would have to be a much larger sum.
Dr Maurice Venning
77. Dr Venning is an assessor and valuer of intangible assets including, and in particular, intellectual property rights in the technology sector. In his statement (A10), he said between 1988 and 1991 inclusive, he was employed by Invetech Operations Pty Ltd. Since 1992, he has carried on business on his own account through his family company.
78. The approach Dr Venning adopts when conducting a valuation includes, early on, as a second step, forming a ballpark preliminary view as to the likely value of the technology. Therefore, when he went to Adelaide on 17 March 1992 to see Dr Smeaton, whom he had met previously in 1989, his stated intention was to form a view as to a ballpark figure for the value of the technology. Dr Venning said that in other valuations he has withdrawn at this early stage if he felt he could not justify a valuation looked for by the parties.
79. The fifth step in Dr Venning's approach is to select the most appropriate valuation methodology or combination thereof based on the available information. In this case he chose to use the discounted cash flow (``DCF'') methodology which was most commonly used at that time, with modifications to address its weaknesses. He rejected the comparables methodology because he was unable to find any comparables. The sixth step in Dr Venning's approach, when an income-based approach is used, is to choose a suitable discount rate. The choice of discount rate reflects the level of risk.
80. Dr Venning was asked about his having given Dr Smeaton comments on a draft letter to the IRDB. Dr Venning said he felt that Bresatec did not understand what was required by the IRDB. Because he knew from experience what the IRDB would be looking for, he offered to help - but he saw this as quite separate from any valuation he would carry out. The additional paragraph suggested by him in the IRDB letter sent to him by Dr Smeaton was one he had taken from another IRDB application.
81. Dr Venning said that on the day of his meeting with Dr Smeaton on 17 March 1992 he was not able to reach a ballpark figure for the valuation of the core technology because Dr Smeaton did not provide him with enough information. Dr Venning could not recall Dr Smeaton telling him what he thought the core technology was worth, nor did Dr Venning get any impression of how much R and D funding Bresatec was seeking. By the time he sent Dr Smeaton the amended draft IRDB letter on 22 April 1992, he felt the core technology was ``good'' and had commercial potential, but he had not formed a view as to a valuation. He was not privy to any discussions between Dr
ATC 2144
Smeaton and Mr Phillips as to the likely amount of funding nor the likely value to be attributed to the core technology.82. Dr Venning said he has no recollection or record of Dr Smeaton telling him that he thought the core technology was worth between $15M and $25M. Dr Smeaton provided him with projected revenue from the sale of transgenic pigs together with other market information. Dr Venning made his own independent enquiries, including of the UK Meat and Livestock Commission. Dr Smeaton's figures seemed reasonable. Dr Venning said, in 1992, he was not aware of transgenic flesh being sold anywhere in the world although plant-based transgenic food was being accepted in the US market. Dr Venning conducted a database search but did not locate any relevant information.
83. Dr Venning met with Dr Smeaton and Dr Robins in Adelaide on 10 June 1992. During the course of the day, he also conducted a database search focusing on the research activities of a number of biotechnology and pharmaceutical companies to see what they were doing in the market. Later that day, he met again with Dr Smeaton and Dr Robins to discuss the results of his search and possible scenarios for commercialisation of the results of the R and D project. At that time, it was Dr Venning's view that a ballpark value for the core technology was between $10M and $19M. Dr Venning was not aware of earlier discussions between MBL and Bresatec suggesting a valuation of around $12.2M.
84. Dr Venning was asked about his projections of revenue flow. He said his figures took into account taxation at 39%. The discount rate he applied, which he considered a fair assessment of the risk ahead having weighed up the issues, was 15.3% post-tax, or about 25% pre-tax. He took into account Cyanamid's investment, the costs of recreating the technology, and the limitations of the DCF approach in valuing long term projects. He considered the 45% pre-tax discount rate applied to pharmaceutical projects requiring regulatory approval was too extreme. He could not recall when he discussed discount rates with Dr Smeaton but agreed this would have been prior to his final report. This would have been in order to explain his report to Dr Smeaton. At no time did Dr Smeaton influence the discount rates he used in the report.
85. Dr Venning said DCF is better applied to short-term rather than long-term projects because it often does not take into account future applications of technology, applications of technology into developing markets, and important strategic considerations. This is why he also used the cost-based methodology as a cross check. Dr Venning said that in 1992 the real options approach was at an early stage of development. He has only been using this approach in the last few years.
86. Dr Venning said he was aware by early June 1992 that the funding required for Bresatec's proposed R and D was $9M. He acknowledged that consumer attitudes towards transgenic meat were unknown at that time, but he considered controversy over the insertion of human genes in cows to increase milk production as being different from a pig gene being inserted in a pig to improve its feed efficiency and bring it to market earlier. He said that even if he had taken greater account of the likelihood of consumer resistance, he doubted this would have changed his valuation.
87. In his statement, Dr Venning said that:
``In my experience, it is impossible to give a definite value to technology. The most that a valuer can responsibly do is to provide a range within which the value of such technology can reasonably be expected to fall.''
(A10, para 43)
In this final valuation, he remained of the view that the value of the core technology was in the range $10M to $19M but said that $15.35M was a reasonable value to assume. In doing so, he considered the costs incurred over the previous ten years in reaching that stage in the research - a figure ``of the order of $15M'' (Tab218, p 28). Overheads, for example associated with the laboratories, were not taken into account in that figure, so it was a conservative one.
Mr Robert Mooney
88. At the relevant time in 1990-1992, Mr Mooney was Vice President, Licensing and Acquisitions, of Cyanamid, a company purchased by American Home Products that ceased to exist on 31 December 1994. Mr Mooney worked for Cyanamid from 1957 until his retirement from American Home Products on 31 March 1995. Initially, on joining Cyanamid after graduating with a Bachelor of
ATC 2145
Science in Biology, he worked as a research scientist.89. In his statement (A11), Mr Mooney said that from the early 1980s, Cyanamid pursued a strategy of external research collaborations either through equity participation or licensing arrangements. One of Cyanamid's ongoing research projects involved porcine somatotropin (``PST''), a naturally occurring protein in pigs which, when injected, causes the pig to grow faster and more efficiently, producing leaner meat at a lower cost to the producer.
90. Mr Mooney learned of Bresatec's research in early 1990 and considered that their transgenic project, involving putting the PST gene directly into the pig cell, to be a superior alternative to Cyanamid's injectable PST project. In about May 1990, he visited Australia and met with Dr Smeaton and members of the research team. By the middle of 1990, Mr Mooney had formed the view that Bresatec's technology was ``more advanced than any comparable transgenic technology in the world'' and that Cyanamid should consider pursuing a strategic equity investment in Bresatec.
91. Mr Mooney said Cyanamid categorised research projects according to five phases: Phase I - early discovery; Phase II - discovery examined for safety and efficacy and compared to competing drugs or technologies, with risk issues being resolved; Phase III - large scale field trials, registration efforts and preparations for first sales; Phase IV - post registration expansion of geographic approvals; and Phase V - technical effort to support on- going sales. Mr Mooney assessed the Bresatec technology as having completed Phase II and being ready to begin Phase III. The safe, controlled and effective expression of the transgene had been demonstrated, and it was ready to be tested on a large scale and for government approval to be sought for sale of the meat for human consumption.
92. On 7 August 1990, Mr Mooney wrote to Dr Smeaton proposing that Cyanamid become an equity partner in Bresatec. Negotiations then proceeded over some months with Mr Mooney being keen to ensure that Cyanamid could gain access to the product of Bresatec's research on the transgenic technology but also, to a lesser extent, to its ESC technology. Ultimately, agreement was reached with the grant of options by Bresatec to Cyanamid as a result of which Cyanamid invested $2M in Bresatec, purchasing approximately 20% of its shares. Mr Mooney stated that (A11, p 13):
``if I had thought that the value of Bresatec's technology was only approximately $11 million (the company's approximate stock value at the time of the investment), I would not have recommended that Cyanamid made the investment. I considered the potential sales of Bresatec's PST transgenic pig technology to be 30% of the global PST market and, therefore, many times greater than $11 million.''
93. Mr Mooney stated during late 1991, he arranged for Dr Smeaton and Dr Wells to meet with pig producers in the US to gauge their interest in purchasing transgenic pigs. The producers were very positive about taking up the technology and Mr Mooney ``considered this to be a clear path to market''.
94. Mr Mooney acknowledged that transgenic animals had not been approved for commercial sale at that time but said, following his discussions with the US Department of Agriculture, he considered the signs were very positive as to the regulatory scheme. Mr Mooney does not know why American Home Products did not exercise the option extended to 1998, to exploit Bresatec's technology by taking a licence. He said that, today, the Department of Agriculture has responsibility for approving the sale of genetically modified organisms, both plant and animal.
95. Mr Mooney agreed that he discussed the proposed R and D Syndicate structure with Dr Smeaton, but did not recall either meeting with or discussing the Syndicate with Mr Phillips of MBL. He attended a Bresatec Board meeting on 20 May 1992 when the Syndicate was discussed, and a copy of the Syndicate documents were forwarded to him and were considered by various Cyanamid departments including the Legal Department:
``The view Cyanamid came to was that its rights under its agreements with Bresatec were not compromised by the syndication. This was because the marketing rights were still to be held by a Bresatec company. In fact, if anything, those rights were enhanced by the value of the research that would be funded by the syndication. If the technology was successful, Cyanamid would fulfill its obligations under its agreement with
ATC 2146
Bresatec and commercialise that technology on a world-wide basis.''(A11, para 72)
Evidence of the expert valuers
96. Apart from Dr Venning, the parties called five expert valuers to give evidence in relation to the valuation of the core technology licence. In summary, the valuers used the following methodologies and reached the following valuations:
-
For the Applicant
- • Dr Venning - modified discounted cash flow (DCF) methodology - $10M to $19M, agreeing that $15.3M is a reasonable value.
- • Mr Todd Morrill - comparables analysis (CA) - a range of $18.2M to $24.8M.
- • Professor Tom Smith - real options analysis using a decision tree analysis approach - a value of $69.66M in a range of $27M to $109M.
-
For the Respondent
- • Mr Kenneth West - critical of Dr Venning's assumptions and of Mr Morrill's comparables, no specific valuation.
- • Mr Wayne Lonergan - DCF - a range of negative $10.31M to negative $1.7M.
- • Professor Kenneth Lehn - DCF - a range of negative $5.4M to positive $3.84M.
The methodologies
97. The three main methodologies utilised by the valuers are, in simplistic terms, as follows:
- • A Discounted Cash Flow (``DCF'') methodology involves estimating the future cash flows of a project or enterprise and applying a discount rate reflecting the level of risk, inflation, time value of money etc, to arrive at a valuation.
- • A Comparables Analysis (``CA'') involves estimating the value of a project or enterprise by considering the value of other similar products or enterprises in the market, having regard to their operations and stage of development.
- • A Real Options Analysis involves a consideration of the options or opportunities which become available in the life of a project or enterprise. One subset of this methodology is a decision tree analysis which considers how decisions might be made at various stages in the project on an assessment of the probability of failure or continuation. The presence of a number of real options results in a range of possible outcomes giving rise to expected cash flows to which a discount rate, reflecting the level of risk, is applied in order to make a valuation.
The expert witnesses are very briefly described below.
Mr Todd Morrill
98. Mr Morrill is the Managing Director of Venture Merchant Group LLC, a San Francisco based private merchant bank. He has extensive experience in venture capital strategic partnering especially in the biotechnology and pharmaceutical industry (A13).
99. Mr Morrill valued the core technology licence using a comparables analysis examining ``the actual market value of technologies and companies to estimate the value of target technology''. He said this approach has significant advantages over any technique relying on future cash flows because it relies on the public market place to set appropriate values. One of the difficulties with biotechnology and pharmaceutical products is that the product often creates the market. Thus, if DCF is used, estimating forward sales is an exercise in guesswork. In this case, having used the comparables valuation methodology, he used capitalised investment to date as a check.
100. Mr Morrill acknowledged that the most significant limitation on his valuation of the core technology licence is the use of a smaller number of comparables than he would have preferred. From a database of 500 companies, using 19 parameters, he identified only nine comparable companies, the critical issue being that of technology comparability.
101. Mr Morrill said that in 1992 he was advising clients that an appropriate return on capital was 20% to 25%. In this case, he chose a venture capital discount rate of 50% as reasonable. Using a comparables analysis, he valued the core technology licence in the range of $20.5M to $27.9M. Using capitalised investment to date, he valued the licence in the range of $15.9M to $21.6M. Thus, the average range is $18.2M to $24.8M, which he stated is a
ATC 2147
conservative and accurate true market value of the licence in June 1992.
Professor Tom Smith
102. Professor Smith is Professor of Finance and Accounting at the Graduate School of Management at the University of NSW (A15). Professor Smith said:
``Although DCF is a generally accepted method of valuation, in this particular case the approach is fundamentally flawed and their valuations should be disregarded. The valuations fail to take account of the real option value in the valuation of Biotechnology applications and hence are grossly understated.''
(A15, para 12)
103. Professor Smith utilised a real options methodology to value the core technology licence at $69.66M, in a range of $27M to $109M. He said a decision tree analysis approach to a real options valuation is considered best practice in biomedical matters and this is the approach he adopted in arriving at what he considered a conservative valuation.
Mr Kenneth West
104. Mr West is the Principal of Technology Commercialisation Group LLC, a firm providing consulting services to the life sciences industry, based in North Carolina (R13). In the late 1980s and early 1990s, Mr West was a business development executive at one of the few then existing biotechnology companies, Embrex Inc. This company had a relationship with Monsanto similar to Bresatec's relationship with Cyanamid. Embrex was involved in researching transgenics in birds, including chickens.
105. Mr West was critical of what he referred to as Dr Venning's unrealistic assumptions of revenues and of the time frame within which they would be generated. He also criticised Mr Morrill's analysis on the basis that none of the comparables selected by Mr Morrill were, in fact, comparable.
Mr Wayne Lonergan
106. Mr Lonergan is Managing Director of Lonergan Edwards & Associates Ltd. He is a chartered accountant and an experienced valuer of R and D projects (R15). He said where a product is not already on the market, he uses a DCF methodology in conducting a valuation and a discount rate of between 30% and 50%.
107. Mr Lonergan assumed that he was required to assess the market value of the core technology licence. He had previously been retained by the Respondent to prepare a list of questions to put to Dr Venning about his valuation in March 2001.
108. Mr Lonergan stated that in his view Dr Venning's valuation of the core technology licence is incorrect and materially overstated. Amongst other shortcomings, it is based on cashflow forecasts which assumed a technical and commercial success for the project, despite a high level of risk. Thus, the discount rate applied by Dr Venning was far too low. Mr Lonergan concluded that the core technology has no commercial value to a third party.
109. Mr Lonergan was also critical of Mr Morrill's valuation, which in his opinion is incorrect and materially overstated. Moreover, Mr Lonergan said Mr Morrill's valuation methodology is flawed because there are no true comparables. His use of cost as a check on value is unreliable because cost is not necessarily related to value.
Professor Kenneth Lehn
110. Professor Lehn is a Professor of Finance in the Katz Graduate School of Business at the University of Pittsburgh, where he teaches graduate courses including in business valuation (R16). He worked for some years as Deputy Chief Economist and then Chief Economist at the Securities and Exchange Commission (``SEC'').
111. Professor Lehn said, in his opinion, DCF is the best valuation methodology. Its shortcomings can be addressed by sensitivity analyses. The comparables method, while easy to use, has the major disadvantage that it is almost impossible to find good comparables, especially with a start up enterprise. The lack of comparable companies is his primary criticism of Mr Morrill's valuation.
112. The main problem with the real options method is that one needs good data that generally does not exist: for example, as to the probability of success or the true costs of obtaining regulatory approval for an unproven drug or other compound. If one inputs bad data, the result is a very distorted and misleading value. In this case, Professor Smith put enormously inflated inputs into the model that gave an extraordinarily high valuation which is simply not credible.
ATC 2148
113. Professor Lehn said the major discrepancy between his valuation and Dr Venning's is the choice of discount rates. The discount rate addresses the risk of the projected cash flows not being achieved. In this case, Professor Lehn applied a discount rate of 40% for the first five years and 20% thereafter. He said this was generous. If he had done sensitivity analyses, he would have used higher discount rates. Venture capital firms typically use discount rates of 40% to 50% to value start up companies. In cross-examination, Professor Lehn acknowledged that the royalty figure he had used for the Syndicate was 15% of 6% of the revenue generated by Bresatec Marketing rather than 15% of the whole revenue (from the first five years of commercialisation) generated by Bresatec Marketing. He agreed that such a correction would lead him to make a substantially higher valuation. His original stated valuation of the core technology licence is a range of negative $5.4M to positive $3.84M (R16, para 115).
Consideration of the law and findings
114. The first issue to be addressed is whether MS3 was entitled to an allowable deduction of $15,196,000 in the financial year to 30 September 1992 pursuant to s 73B(12) .
115. The Tribunal finds that MS3 paid $15,196,000 as contribution to the core technology licence fee of $15,350,000 paid to Lumunis by the Syndicate. The Syndicate thereby acquired a licence to use the intellectual property to which Luminis was then entitled relating to transgenic pigs and ESC technology. The expenditure, being part of a series of transaction arrangements, enabled Bresatec to pursue its R and D on transgenic pigs and ESC technology with a view, in particular, to producing a fast growing, more food efficient and leaner pig. Pursuant to the definitions of ``core technology'' and ``core technology expenditure'' in s 73B(1AB) and s 73B(1) set out above, the Tribunal determines that MS3 incurred core technology expenditure of $15,196,000 in the financial year to 30 September 1992 and, prima facie, was entitled to an allowable deduction of that amount in that year of income under s 73B(12) of the Act.
116. The second issue is whether MS3 was entitled to an allowable deduction of $178,098 in respect of interest paid by MS3 to MAL in that year of income .
117. As stated above, s 73B(14) permits a deduction in respect of R and D expenditure where the aggregate amount is greater than $20,000. The $178,098 interest paid by MS3 to MAL was in respect of the debt funding of $10,890,000 borrowed by MS3 from MAL to complete the transaction arrangements enabling the R and D expenditure. Thus, prima facie, MS3 was entitled to such a deduction in that year of income. The Tribunal notes that, pursuant to s 73B(34), the IRDB issued a letter dated 16 June 1992 confirming its determination that the then proposed project satisfied the definition of R and D under s 73B(1) of the Act and that the Board had registered the Syndicate for the years 1991/1992, 1992/1993, and 1993/1994 financial years (C108).
118. Having made those preliminary determinations, the Tribunal must then consider whether the anti-avoidance provisions relied on by the Respondent apply. The first of these is s 73B(31). As stated, this subsection gives rise, in turn, to two further questions.
119. First, having regard to any connection between the Syndicate and Luminis and to any other relevant circumstances, is the Tribunal satisfied that the Syndicate and Luminis ``were not dealing with each other at arm's length in relation to the incurring of that expenditure''?
120. The parties made lengthy submissions on the meaning of dealing at arm's length and referred the Tribunal to a number of authorities. In
Australian Trade Commission v WA Meat Exports Pty Ltd (1987) 75 ALR 287, the Full Federal Court, at 291, referred to the explanation in Black's Law Dictionary and, in particular, to the parties acting in their own self- interest, in a bargained transaction. In
Barnsdall v FC of T 88 ATC 4565; (1988) 81 ALR 173, Davies J, at ATC 4567-4568; ALR 176-177, emphasised the distinction between whether the parties are at arm's length and whether their dealing was at arm's length. Notwithstanding that there is a close relationship between the parties, there may still be transactions where they deal with each other at arm's length. In
The Trustee for the Estate of the late AW Furse No. 5 Will Trust v FC of T 91 ATC 4007 at 4015, Hill J said:
``What is required in determining whether parties dealt with each other in respect of a particular dealing at arm's length is an
ATC 2149
assessment whether in respect of that dealing they dealt with each other as arm's length parties would normally do, so that the outcome of their dealing is a matter of real bargaining.''
(See also Hill J in
Copperart Pty Ltd v FC of T 93 ATC 4779 at 4799.)
121. In
Granby Pty Ltd v FC of T 95 ATC 4240 at 4243-4244; (1995) 129 ALR 503 at 507, Lee J said:
``... the term `at arm's length' means, at least, that the parties to a transaction have acted severally and independently in forming their bargain. Whether parties not at arm's length have dealt with each other at arm's length will be a matter of fact...
If the parties to the transaction are at arm's length it will follow, usually, that the parties will have dealt with each other at arm's length. That is, the separate minds and wills of the parties will be applied to the bargaining process whatever the outcome of the bargain may be.
That is not to say, however, that parties at arm's length will be dealing with each other at arm's length in a transaction in which they collude to achieve a particular result, or in which one of the parties submits the exercise of its will to the dictation of the other, perhaps, to promote the interests of the other.''
122. Jenkinson J quoted this with approval in
Collis v FC for T 96 ATC 4831 at 4837. In that case, there was no error of law by the Tribunal, which found that the indifference of one of the parties to a transaction lead to a conclusion that the parties were not dealing with each other at arm's length. The party ``submitted the exercise of his will to the applicant's wishes''. (See also
Pontifex Jewellers (Wholesale) Pty Ltd v FC of T 99 ATC 5324 at 5327-5328.)
123. In the present case, there is no dispute that the parties to the principal transaction, that is MS3 and Luminis, were at arm's length. For both parties, this was a commercial relationship. At issue, however, is whether the dealing between them, namely MS3's purchase (through the Syndicate) of the core technology licence from Luminis, was at arm's length.
124. The Tribunal rejects the Applicant's submission that it follows from Lee J's exposition of the law in Granby (supra) that, where the parties are at arm's length, there is a presumption that their dealing was at arm's length. In the Tribunal's view, it is a question of fact in each case as to whether the parties acted independently and applied their separate minds and wills in forming a bargain.
125. The focus of much of the evidence was the fee paid for the core technology and its valuation. The Respondent submits that MBL was indifferent to increases in the value attributed to the core technology licence and, indeed, that such increases were to MBL's benefit as it would enlarge its tax benefit. Secondly, the Respondent submits that there was no arm's length bargaining over the licence fee. The amount of the fee was increased in order to provide additional returns to MBL, when MBL assumed the risk of a change in the rate of company tax. Thirdly, the fee was not fixed by a process of independent valuation? Dr Venning acquiesced in amounts put to him by Dr Smeaton and Mr Phillips.
126. The Respondent submits (Respondent's submissions p 5) that:
``The valuation was not independent, but was reached by a process of persuasion and cooperation amounting to collusion between parties with a common interest in maximising the value to be attributed to the core technology.''
127. The Applicant contends that there is no credible suggestion that the will of either of the parties was suborned to the will of the other, nor that they acted in concert or for an ulterior purpose. Bresatec's and Luminis' objective was to obtain R and D funding with a view to commercialisation. MBL's objective was to build a portfolio of high quality technology project assets while taking advantage of specific government incentives to protect its investment if the R and D was not successful.
128. The Applicant points to the negotiations over the price to be paid for the licence and, in particular, to Dr Smeaton's and Mr Phillips' evidence about this. Secondly, the licence fee was part of a package that was extensively negotiated. Thirdly, the fact that the parties agreed to a particular valuation does not mean they were acting in collusion or suborning their interests one to the other.
129. The Tribunal notes that the first approach to MBL was made by Dr Gregson of Hambro-Grantham, a director of Bresatec, enquiring whether MBL would be interested in
ATC 2150
an R and D syndication. Evidence would suggest that this was in early to mid 1991 (A9). The transaction was not concluded until 30 June 1991/1 July 1992. Over that period, four indicative proposals were made by MBL, including two that were made in late July/early August 1991 (C1 and C2) before Mr Phillips' involvement with the Syndicate. It is clear from the evidence of the parties, including that of Dr Smeaton, Mr Phillips and Mr Hart, and from the documentary evidence, that there were extensive discussions between Bresatec and MBL over arrangements for the transaction and its terms. The Tribunal has no hesitation in finding that the transaction between MBL and Bresatec was, as a whole, conducted at arm's length.130. However, a more specific examination must be made of the parties' dealings with regard to the core technology licence fee and the R and D expenditure, which are at issue in relation to s 73B(31)(b)(i). Dr Smeaton's evidence was that he always considered Bresatec's transgenic technology to be worth between $15M and $25M, and that he continued to express this view throughout the negotiations leading to the conclusion of the transaction. When he first met Mr Phillips on 12 December 1991, he told him of his view and of the Cyanamid agreement which valued the shares in Bresatec at about $12M. It was after this meeting, on 16 December 1991, that Mr Phillips sent Dr Smeaton a modified indicative proposal assuming a value of $12.2M for the Bresatec core technology (C20). Mr Phillips seems to have arrived at this figure on the basis of Dr Smeaton telling him Bresatec was seeking $9M in R and D funding, on the comfort he derived from the Cyanamid agreement, and on his rule of thumb that R and D funding should be 60% to 70% of the value of the core technology (A9, para 30). Mr Phillips had, however, told Dr Smeaton at the meeting that an independent valuation would be required. At this stage, the figure for the core technology was still only an assumed value and Dr Venning had not yet finalised his valuation.
131. The Tribunal finds that Dr Venning acted independently in preparing his valuation. As a matter of common sense, it was necessary for him to have ongoing communication with Dr Smeaton because he was the main source of information about Bresatec required by Dr Venning in the preparation of his report. Dr Smeaton gave evidence that he formed the view, after his meeting with Dr Venning on 17 March 1992, that Dr Venning would take an independent view in making his valuation. Mr Phillips said that in the valuation Dr Venning had prepared for MBL previously in relation to another matter, he had been impressed by, amongst other things, Dr Venning being protective of his independence. Mr Phillips said he would have rejected Dr Venning's report if he had thought it was not independent.
132. Dr Venning also impressed the Tribunal as a person who guarded his independence and sought to form his own independent view. The Tribunal accepts that the assistance he gave Dr Smeaton in looking over the draft IRDB letter and suggesting an additional paragraph from a previous IRBD application, was a separate matter and did not compromise his independence. Ultimately, Dr Venning's valuation was that the core technology was worth between $10M and $19M, and that a valuation of $15M was reasonable. The fact that he amended this figure from $15M to $15.35M after discussion with Mr Phillips does not, in the Tribunal's view, compromise his valuation. He said $15.35M was within his range of values and he considered this reasonable. The Tribunal notes, as the expert witnesses made clear, that valuation is often not a precise science especially where projections for the development and commercialisation of biotechnology are concerned.
133. The Tribunal rejects the Respondent's submission that there was persuasion and cooperation amounting to collusion between the parties. It notes, in passing, that persuasion is part of the normal process of negotiation and that cooperation does not, of itself, indicate that parties fail to exercise their separate minds and wills in reaching a bargain. The Tribunal also rejects the Respondent's submission of indifference on MBL's part. Mr Phillips' evidence and the process of negotiation between the parties indicates that this was not the case.
134. The Tribunal therefore concludes that the parties applied their separate minds and wills in forming a bargain and that their dealing over the relevant expenditure was at arm's length. Having so determined, it is not necessary for the Tribunal to consider the second question raised by s 73B(31)(b)(ii), namely whether the Tribunal is satisfied that the
ATC 2151
amount of that expenditure would have been less had the parties been dealing with each other at arm's length in relation to incurring that expenditure. However, in the light of the Part IVA issues to be addressed, it seems appropriate to at least briefly consider the valuation evidence.135. The Tribunal heard evidence from Dr Venning and five expert witnesses on the question of the valuation of the core technology. While it seems the DCF methodology is the most widely accepted, the comparables and real options methodologies also have their advocates, and each has its limitations. Obviously, in the case of new technology requiring further R and D and commercialisation, where there is no clear market or income stream, no valuation can do more than estimate a range within which a particular valuation may reasonably lie. As the Applicant submitted, ``there was no absolutely correct price for the technology'' (Applicant's Submissions in reply, para 9). It is also worth noting that the five expert witnesses had the benefit of hindsight and that it is easy to be critical after the event when what was then unknown is now known. In the context of what was no more than an estimate of value, care needs to be taken when substituting one valuer's opinion for another without good reason.
136. With regard to s 73B(31)(b)(ii), it should be noted that the relevant question is what would the expenditure have been if these parties had been dealing with each other at arm's length? Would a competent and independent valuer other than Dr Venning have made a different valuation which the parties would have relied on in concluding the terms of transaction so that the amount of the relevant expenditure would have been less?
137. The Tribunal finds that Dr Venning acted bona fide in making an independent valuation. He is a Doctor of Philosophy in Microbiology as well as having a Bachelor of Economics degree. The Tribunal accepts that his scientific background facilitated his understanding of the transgenic technology developed by Bresatec and of the ESC technology developed by the University's research scientists, the intellectual property in which was vested in Luminis. The Respondent's expert witnesses were critical of Dr Venning's assumption of success for the project, of his optimistic projections of future revenue from commercialisation, and of his appreciation of what they considered to be an unrealistically low discount rate, thereby recognising a low level of risk. The Tribunal notes that Mr Lonergan proceeded on the basis that he was assessing the market value of the core technology licence. While he is undoubtedly an experienced valuer, the Tribunal's impression, in terms of his adjustments to the projected revenues and choice of discount rate, is that he was unduly pessimistic about the future of the project. Certainly he did not have the scientific understanding of the technology that Dr Venning had. Mr Lonergan also criticised Dr Venning's projection for penetration of the US market without, apparently, giving credence to Dr Venning having based his projection of 30% penetration on Mr Mooney's figures (Transcript, 16 August 2002, p 853).
138. The Respondent's witnesses were also critical of Mr Morrill's valuation on the basis that the comparables he selected were not, in fact, comparable. Professor Smith was criticised by Professor Lehn for the use of ``inflated DCF inputs'' in his analysis that gave rise to a value ``drastically higher'' than the other values recorded and ``simply not credible'' (R17, para 31). Certainly, Professor Smith's valuation is significantly higher than the others. However, there was also an error in Professor Lehn's calculations: he used a royalty figure for the Syndicate of 15% of 6% of the revenue generated by Bresatec Marketing rather than 15% of the whole of the revenue generated when calculating his Exhibits I1 - I8 (Transcript 16 August 2002, p 872).
139. The conclusion reached by the Tribunal in the light of Dr Smeaton's view of the worth of the technology, and of the value attributable to Bresatec by virtue of Cyanamid's investment in its shares, is that a competent and independent valuer other than Dr Venning might have made a similar valuation. The Tribunal is not satisfied that his valuation, which was conducted using the most widely accepted methodology, was flawed such that the Tribunal should substitute a different valuation. The Tribunal finds, having regard to the matters set out in s 73B(31)(c), (d) and (e), that Dr Venning acted professionally, from an informed scientific and economic background, and that there is insufficient evidence to suggest
ATC 2152
that the relevant expenditure would have been any less had the valuation been made by a different competent and independent valuer undertaking the valuation in 1992.140. The Tribunal must next consider the second of the anti-avoidance provisions relied on by the Respondent, contained in Part IVA of the Act, namely s 177D. The Tribunal notes that there are three principal issues to be addressed. First, was there a ``scheme'' within the meaning of Part IVA?
141. The definition of ``scheme'' in s 177A(1) appears above. The parties did not dispute that there was a scheme within the meaning of the definition. In the latter stages of the hearing there was, however, disagreement between the parties over the particularisation of the scheme by the Respondent and which tax benefits derived from the scheme were at issue in these proceedings. Initially, the Respondent only identified two tax deductions: the core technology licence fee and the interest component of the R and D. Latterly, the Respondent sought to identify other tax benefits as part of the scheme, in particular, the R and D expenditure in relation to MS3 and benefits accruing to Macquarie Finance. Ultimately, the parties came to an understanding, with the Respondent giving assurances that there would be no further determinations made giving rise to adjustments in relation to the other tax benefits (Transcript 21 August 2002, p 1060). (See also
FC of T v Peabody 94 ATC 4663 at 4669-4670; (1994) 181 CLR 359 at 382-383.)
142. The second issue is whether a taxpayer obtained a tax benefit in relation to the scheme . ``Obtaining a tax benefit'' is defined in s 177C(1) which includes, relevantly, a deduction being allowable to the taxpayer in relation to a year of income where the whole or a part of that deduction would not have been allowable to the taxpayer in relation to that year of income if the scheme had not been entered into or carried out (s 177C(1)(a)).
143. The Applicant does not dispute that the transaction was structured in a way that enabled MS3 to obtain the deductions for which the Act specifically provided (Applicant's Submissions, p 46, para 186). The Applicant's submissions accept that the tax deductions which are the subject of these proceedings constituted a tax benefit to MS3. The Applicant's objection in the latter stages of the hearing was to the Respondent changing its particularisation of the scheme by including reference to tax benefits accruing to Macquarie Finance. In the Tribunal's view, the more important question is the dominant purpose of the scheme.
144. In his submissions, the Respondent submitted that the allowable deduction of $15,196,500 claimed by MS3 in respect of the acquisition of the core technology licence by the Syndicate from Luminis was a tax benefit to MS3 in connection with the scheme in the subject year of income, within the definition of tax benefit in s 177C(1)(b). The Respondent further submitted that the allowable deduction of $178,098 claimed by MS3 was core technology expenditure, being interest referable to that part of a loan from MAL to MS3 that was used by MS3 in connection with the acquisition of the core technology licence from Luminis for $15,196,500, and a tax benefit to MS3 in connection with the scheme in the subject year of income, as defined in s 177C(1)(b).
145. These submissions were not contested by the Applicant (Applicant's Submissions, p 33, para 129) and, as the Respondent noted, it is difficult to foresee any circumstances in which MS3 would have incurred such expenditure other than pursuant to the scheme. The Tribunal therefore finds that the taxpayer did obtain a tax benefit in relation to the scheme.
146. The third issue is whether, having regard to the matters set out in subparagraphs (i) to (viii) of s 177D(b), it would be concluded that the person or persons who entered into or carried out the scheme or any part of the scheme did so for the purpose of enabling the relevant taxpayer to obtain a tax benefit .
147. The application of s 177D(b) has been the subject of interpretation by the Federal and High Courts. In
Peabody v FC of T 93 ATC 4104 at 4113; (1993) 40 FCR 531 at 542, Hill J (with whom Ryan and Cooper JJ agreed) said the purpose to which the section refers may be either the sole or dominant purpose, objectively determined:
``It will be seen that the determination of what schemes fall within s 177D requires an objective conclusion to be drawn, having regard to the matters referred to in para (b) of the section, but no other matters. It is notable that the actual subjective purpose of any relevant person is not a matter to which
ATC 2153
regard may be had in drawing the conclusion......
It will be seen, from s 177D, that the conclusion that is required to be drawn is not a conclusion with respect to the scheme itself, but a conclusion as to the purpose of a particular person.''
148. In
FC of T v Spotless Services Limited & Anor 96 ATC 5201; (1996) 186 CLR 404, the High Court (excepting McHugh J who, while agreeing with the other six judges, delivered a short separate judgment) said, at ATC 5206; CLR 416:
``... In its ordinary meaning, dominant indicates that purpose which was the ruling, prevailing, or most influential purpose.''
The Court had noted earlier, at ATC 5206; CLR 415:
``A person may enter into or carry out a scheme, within the meaning of Pt IVA, for the dominant purpose of enabling the relevant taxpayer to obtain a tax benefit where that dominant purpose is consistent with the pursuit of commercial gain in the course of carrying on a business.''
The Court concluded (at ATC 5210; CLR 422) that, in that case:
``... the question is whether, having regard, as objective facts, to the matters answering the description in par (b), a reasonable person would conclude that the taxpayers entered into or carried out the scheme for the dominant purpose of enabling the taxpayers to obtain a tax benefit in connection with the scheme.''
149. These principles were recently again endorsed by the Full Federal Court in
Hart & Anor v FC of T 2002 ATC 4608; [2002] FCAFC 222. In his exposition of the relevant authorities, Hill J compared
Eastern Nitrogen Ltd v FC of T 2001 ATC 4164; [2001] FCA 366 and
FC of T v Metal Manufactures Ltd 2001 ATC 4152 with the High Court decision in Spotless (supra). At paragraph 60, he said that in the first two cases [at 4622]:
``... it is hard to imagine that the transaction would have been structured in the way it was... but for tax. Yet it was held that the scheme was not one to which Part IVA applied. Special leave to appeal this decision was refused by the High Court.''
He emphasised that it is a matter of what view the reasonable person would form of the scheme.
150. In a separate judgment, Hely J, who agreed with Hill J's conclusion in relation to Part IVA, made some additional observations [at 4625-4626]:
``81. A particular course of action may be both tax driven, and bear the character of a rational commercial decision. The presence of the latter characteristic does not determine in favour of the taxpayer whether, within the meaning of Part IVA, a person entered into or carried out a `scheme' for the dominant purpose of enabling a taxpayer to obtain a tax benefit:
FC of T v Spotless Services Limited & Anor 96 ATC 5201 at 5206; (1996) 186 CLR 404 at 416. But nor does the fact that a taxpayer adopted one of two or more alternative courses of action, being the one that produces a tax benefit, determine the answer to that question in favour of the Commissioner:
Metal Manufactures Ltd v FC of T 99 ATC 5229 at 5275 per Emmett J (on appeal
2001 ATC 4152; (2001) 108 FCR 150); Spotless (supra) at ATC 5212; CLR 425 per McHugh J;
Inland Revenue Commissioners v Brebner [1967] 2 AC 18 at 30, per Lord Upjohn.82. In construing Part IVA, it is appropriate to bear in mind, as an interpretative aid, the Treasurer's statement in his second reading speech at the time of the Bill for the introduction of Part IVA into the Act, when the Treasurer stated:
`We are acutely aware that the term ``tax avoidance'' means different things to different people.
Reasonable men and women are bound to differ on this crucial question and on the subsidiary matter of the appropriate tests for determining what behaviour a general anti-avoidance provision ought to prescribe. The proposed provisions - embodied in a new Part IVA of the Income Tax Assessment Act - seek to give effect to a policy that such measures ought to strike down blatant, artificial or contrived arrangements, but not cast unnecessary inhibitions on normal commercial transactions by which taxpayers legitimately take advantage of opportunities available for the
ATC 2154
arrangement of their affairs... Some writers on the subject suggest that tax avoidance involves conduct entered into for the sole or dominant purpose of obtaining a particular tax advantage.That description could be expected to cover the types of tax avoidance that, again using the language of social or political debate, are blatant, artificial or contrived, and which are indeed intended to be covered by this Bill.
But it is also apt to describe other arrangements, including some family arrangements, which are beyond the appropriate scope of general anti- avoidance measures and ought, if need be, to be dealt with by specific measures.
In order to confine the scope of the proposed provisions to schemes of the ``blatant'' or ``paper'' variety, the measures in this Bill are expressed so as to render ineffective a scheme whereby a tax benefit is obtained and an objective examination, having regard to the scheme itself and to its surrounding circumstances and practicable results, leads to the conclusion that the scheme was entered into for the sole or dominant purpose of obtaining a tax benefit.'
83. However, the decision in Spotless makes it clear that Part IVA can apply to real commercial transactions. Where the line is to be drawn is a matter of degree, having regard to the eight factors itemised in s 177D.''
151. Turning to the present case, the issue, as stated above, is whether having regard to the matters set out in subparagraphs (i) to (viii) of s 177D(b), a reasonable person would conclude that the taxpayers entered into or carried out the scheme with the dominant purpose of obtaining a tax benefit in connection with the scheme.
152. The Tribunal notes that in Hart (supra), Hill J said at paragraph 53 [at 4620]:
``53. While it is necessary for the Judge hearing the matter to consider each of the listed factors in s 177D, it is unnecessary for him or her in the judgment to deal individually with each and a global assessment of purpose will suffice:
FC of T v Consolidated Press Holdings Ltd & Anor 2001 ATC 4343 at 4360 [94]; (2001) 179 ALR 625 at [94]. It may be noted here that his Honour considered each of the listed factors.''
153. Relying on the evidence of Dr Smeaton, Dr Seamark and Mr Hart, the Tribunal finds that Bresatec's objective, and that of Luminis, was to raise R and D funding to finance ongoing research on transgenic pigs and ESC technology. Dr Smeaton, as Managing Director/ Chief Executive Officer of Bresatec, was focused on raising $9M to enable the further development of the technology in preparation for commercialisation. He had explored various avenues to raise the necessary funds, but whilst he had successfully attracted equity partners in 1989 (Hambro-Grantham and Cambooya) and in March 1992 (when Cyanamid exercised its option dated 1 April 1991 to purchase shares in Bresatec), he had concluded that he would be unable to raise additional funds from existing shareholders. The Tribunal notes Mr Hart's evidence that it was not Luminis' role to provide research funding.
154. The Tribunal notes that it was Dr Gregson of Hambro-Grantham, the Chair of Bresatec's funding committee, who first approached MBL in about July 1991 ``enquiring into the possibility of putting together an R&D Syndicate with the Bank'' (Phillips A9, para 35; Smeaton A8, para 97). Following this approach, MBL sent two indicative proposals to Bresatec in July 1991 (undated letter - C1) and on 9 August 1991 (C3). According to Dr Smeaton, during August and September 1991, he and Dr Gregson attended meetings in Sydney ``with representatives of MBL, BT, Bain and the Commonwealth Bank to discuss the possibility of an R&D Syndicate'' (A8, para 110). In the course of their meetings with MBL, they met Mr Nick Lattimore whom they asked to send a fresh (second) proposal. During September 1991, Dr Smeaton was engaged in meetings with pig producers overseas. In early October 1991, Dr Smeaton travelled to England to meet with a biotechnology company in Cambridge to discuss the possibility of a collaborative agreement which, ultimately, did not eventuate.
155. On or about 15 October 1991, Dr Smeaton received a fax from Mr Lattimore at MBL concerning MBL's successful conclusion of an R and D syndicate with another company (Paxus) on which Dr Smeaton reported to the Bresatec Board. In late November 1991, Dr
ATC 2155
Smeaton received a telephone call from Mr Phillips following which they met in Adelaide to discuss funding through an R and D syndicate.156. Mr Phillips was employed in the Corporate Finance and Leasing Services Division at MBL. He stated that in late 1991 he was asked ``to assume responsibility for putting together a portfolio of R & D investments for the Bank'' (A9, para 5). He saw an opportunity for MBL in the relatively underdeveloped technology sector in Australia and set about building a presence for MBL in that sector:
``Once that presence had been established, I believed that it would be possible for the Bank to carefully select a portfolio of projects that were the most likely to provide the highest returns. It was my intention to attempt to spread the Bank's investments across a portfolio of diverse, high quality and innovative R & D Projects.''
(A9, para 9)
He said that he understood the Federal Government wished to encourage syndicated R and D as a means of attracting private capital for innovative projects.
157. In selecting suitable projects, he looked for researchers who were recognised leaders in their fields and had achieved international recognition. He also looked to see if experienced business people had already invested in the project and how much had been spent in developing the technology. He relied on the IRDB to confirm the appropriateness of the proposed R and D and that suitable arrangements were in place for commercialisation. He relied on the ATO for confirmation that the structure of the proposed syndicate was satisfactory. Finally, he relied on an independent valuer for an independent evaluation of the technology and of the project and for a fair valuation of the market value of the technology.
158. Mr Phillips said despite the investment not being ``at risk'', it was not MBL's practice to pay more for an asset than it was worth. On the other hand, he did not want to take advantage of researchers who were in need of funding because he ``was hoping to build a long term business relationship. I could only do that if there was trust between us'' (A9, para 28). Nevertheless, he included a put option in MBL's R and D investments so that the investments were not at risk and MBL could exit the transaction.
159. The Tribunal notes that s 73CA was inserted in the Act by the Taxation Laws Amendment Act 1990, which took effect on 7 June 1990. Prior to this amendment taking effect, deductions of 150% were permitted for investors in R and D syndicates in recognition of the high degree of risk involved in such investment (1991 Australian Master Tax Guide, para 20-225). However, as investors found ways to lessen the degree of risk, the Government responded by introducing s 73CA which permits a lesser deduction of 100% in respect of R and D expenditure where the investor's funds are not at risk (Commonwealth Parliament, House of Representatives, Taxation Laws Amendment Bill 1990, Explanatory Memorandum, clause 9). In introducing the Bill to effect the amendment, the Minister assisting the Treasurer said:
``Deductions at the rate of 150 percent are now available where all or some funds may be invested at less than full risk. The Government considers that the highest rate of deduction should only be applicable where all of an investor's funds are at risk. New provisions are therefore proposed by the Bill which will reduce the deduction allowable proportionately, from 150 per cent to 100 per cent, as the element of risk is reduced.''
(Commonwealth Parliament, House of Representatives, Hansard No 1, 1990: 9 May 1990, p 181.)
160. In his statement (A9, para 16), Mr Phillips said that in investing in a portfolio of R and D projects for MBL, he ``chose that the investment should not be at risk even though this resulted in a lower level of tax concession on the expenditure''. The structure he used so that the investment was not at risk was to incorporate a put option in the transaction so that at a specified review date, MBL and/or the other investors could require the researcher to purchase MBL's and/or the other investors' interest in the syndicate. The review date was set at a sufficient time in the future to allow effective commercialisation if the research was technically successful.
161. Mr Philips stated that he understood from conversations with officers at the ATO, that the ATO approved the use of put options in this way, provided that there was a reasonable
ATC 2156
prospect of commercialisation and that the commercialisation period allowed, for example, five years, was reasonable (A9, para 18). On 14 July 1992, Mr Phillips lodged an application with the Respondent for an advance opinion in respect of the Syndicate (C132). On 28 August 1992, the Respondent issued a final advance opinion (C134). The fact that the Syndicate's going ahead was conditional upon a favourable ruling is an indication of the importance of the tax deductions to MBL but is not necessarily evidence of dominant purpose.162. At the time the transaction was being negotiated between MBL and Bresatec in late 1991/early 1992, there is no doubt that University of Adelaide research scientists such as Dr Seamark, and Bresatec employees such as Dr Robins and Dr Smeaton, were optimistic about the transgenic and ESC technology and the chances of its successful commercialisation. At that time, while some concerns had been expressed about the consumption of genetically modified food, the Tribunal finds that Bresatec was optimistic that any hurdles could be overcome and regulatory approval obtained. This was because Bresatec's transgenic technology only involved modifying the pig's DNA by inserting an additional pig gene which could promote faster growth, rather than incorporating foreign genetic material.
163. Again, while it is easy to be sceptical in hindsight, the Tribunal is not satisfied that there is any evidence to suggest MBL's motives were other than as stated by Mr Phillips. For example, Mr Phillips said he attended and chaired all but one of the quarterly meetings held in the period 13 January 1993 to 21 July 1995. In the Tribunal's view, such conduct is consonant with this being a genuine investment by MBL. With the benefit of the knowledge that the Syndicate was not ultimately successful, the Respondent has sought to impute a different motive to MBL from the terms of the transaction. However, it is clear from Dr Smeaton's evidence that the R and D was a technical success and that it was only when regulatory approval was not forthcoming in 1995/1996 that commercialisation was put on hold. The Tribunal notes Dr Smeaton's evidence that, in 1999, the Food Acts were modified to allow the sale of genetically modified organisms and that, in 2001, Bresatec subsequently signed an agreement with an international pig company to continue its research (A8, para 354).
164. Mr Phillips' evidence is that he
``personally monitored the progress of the R & D by establishing and chairing Technical Review Committees (TRC) for most of the syndicates included in the Portfolio. In addition, the Bank retained and paid for technical consultants to assist us by attending the quarterly TRC meetings and preparing detailed progress reports for the projects.''
(A9, para 112)
165. Specifically, in relation to the Syndicate in this case, Mr Phillips stated (A9, para 113):
``So that I could review the ongoing research being undertaken by the Bresatec Syndicate and monitor the cost of that research, I chaired quarterly TRC meetings, the members of which included Mr Hart of Luminis and representatives of Bresatec such as Dr Allan Robins (who was responsible for supervising the R & D Project) and Dr Smeaton. I also appointed Dr Venning as the Bank's technical consultant to attend these meetings and to assist the Bank in technical aspects of the research, including the review of the research. The purpose of these TRC meetings was to review the research undertaken during the preceding quarter, to review the progress of that research against the goals and milestones of the R & D Project and to discuss the proposed research for the following quarter. I also chaired quarterly meetings of Investors which generally consisted of the same members as the TRC and were held immediately following the TRC meetings. The purpose of the Investor meetings was generally to review the project expenditure budget and the R & D expenses incurred in the preceding quarter and to monitor the Bank's investment.''
Mr Phillips said he attended and chaired all but one of the quarterly meetings held in the period 13 January 1993 to 21 July 1995.
166. Turning specifically to the matters referred to in subparagraphs (i) to (viii) of s 177D(b), these have already largely been canvassed above, including the manner in which the scheme was entered into and carried out (subparagraph (i)). While the Respondent in its written submission pointed to significant
ATC 2157
differences between the Syndicate arrangements and the model set out in Taxation Ruling IT 2635 (A60), as the Ruling states, Income Tax Rulings do not have the force of law and ATO decisions are made on the merits of each individual case. In this case, MBL obtained an advance ruling from the ATO dated 27 August 1992, which dealt with the 24 propositions put forward by MBL (A134). The Respondent submitted that the transaction documents were designed against the background that there was little possibility that MAL would not exercise its put option against Bresatec. In the Tribunal's view, noted above, such a claim can only be made in hindsight. At the time the transaction was entered into in 1992, the parties were optimistic about the success of the project.167. With regard to subparagraph (ii), the form and substance of the scheme, this has already been discussed at some length above. As to the time at which the scheme was entered into and the period of the scheme (subparagraph (iii)), once again those details are set out above. However, as the Applicant submitted, it should be noted that it was Government policy at the time the scheme was entered into in 1992 to encourage investment in R and D syndicates. Incentives, in the form of tax deductions, were provided for private investors. In this case, the transaction was concluded after a period of almost 12 months from the first contact between Bresatec and MBL, during which time there was a lengthy process of discussion and negotiation.
168. The result achieved, but for the operation of Part IVA (subparagraph (iv)), is the deductions under challenge. The relevant taxpayer - MS3 - will incur a substantial loss in the subject year of income as a result of the scheme (subparagraph (v)). The scheme involved a number of other parties (subparagraph (vi)), detailed above, including Luminis which, like Bresatec, originally owned part of the relevant intellectual property and, unlike Bresatec, had the benefit of tax-exempt status, MAL which funded MS3's participation in the syndicate and, one assumes, would have been liable to pay tax on the interest earned, Macquarie Finance which acted as the deposit holder and, presumably, reinvested the money deposited to earn interest, thereby incurring a tax liability, and Bresatec which conducted the research and, ultimately, was called upon to honour the put option.
169. There do not appear to be any other relevant consequences (subparagraph (vii)), and the connection (subparagraph (viii)) between the parties identified above in relation to subparagraph (vi) is already plain from the description of the transaction.
170. In conclusion, the Tribunal finds that the tax deductions facilitated by s 73B and s 73CA of the Act were important influences on how the scheme was structured, and on MBL through the involvement of its wholly owned subsidiary companies. Clearly, the Government intended tax deductions to be an incentive to encourage private investment in R and D. However, while obtaining a tax benefit in connection with the scheme was undoubtedly an important purpose of the scheme, the Tribunal finds that a reasonable person would conclude that it was not the dominant purpose.
171. MBL is in the business of banking, finance and investment. In this case, it decided to invest in the Syndicate and, in the Tribunal's view, the evidence supports a finding that this investment was its dominant purpose. The extent of Mr Phillips' involvement in the project both in the period before the finalisation of the terms of the transaction on 30 June 1992/1 July 1992 and in the three year period afterwards, and his stated reasons for this, described above, supports this conclusion. Mr Phillips' evidence is, in turn, supported by that of Dr Smeaton and the Applicant's other witnesses who attested to the Bresatec team's optimism about the successful commercialisation of the transgenic pig technology. The events under consideration took place against the backdrop of the Government's tax incentives for such investments. As Hely J recognised in Hart (supra) at paragraph 83 [at 4626], ``Where the line is to be drawn is a matter of degree, having regard to the eight factors itemised in s 177D''. Having had regard to those factors, the Tribunal finds in favour of the Applicant.
172. The Tribunal therefore sets aside the decision under review and substitutes a new decision allowing the Applicant's objection against the Income Tax Assessment dated 14 November 2000 for the Substituted Accounting Period ending on 30 September 1992.
Disclaimer and notice of copyright applicable to materials provided by CCH Australia Limited
CCH Australia Limited ("CCH") believes that all information which it has provided in this site is accurate and reliable, but gives no warranty of accuracy or reliability of such information to the reader or any third party. The information provided by CCH is not legal or professional advice. To the extent permitted by law, no responsibility for damages or loss arising in any way out of or in connection with or incidental to any errors or omissions in any information provided is accepted by CCH or by persons involved in the preparation and provision of the information, whether arising from negligence or otherwise, from the use of or results obtained from information supplied by CCH.
The information provided by CCH includes history notes and other value-added features which are subject to CCH copyright. No CCH material may be copied, reproduced, republished, uploaded, posted, transmitted, or distributed in any way, except that you may download one copy for your personal use only, provided you keep intact all copyright and other proprietary notices. In particular, the reproduction of any part of the information for sale or incorporation in any product intended for sale is prohibited without CCH's prior consent.
