SST 12
Sales tax: classification of drugs, medicines and sunscreen preparations
-
Please note that the PDF version is the authorised consolidated version of this ruling and amending notices.This document has been Withdrawn.View the Withdrawal notice for this document.
FOI status:
may be releasedFOI number: I 1017667| subject | paragraph |
|---|---|
| Chapter 1 - What this Ruling is about | |
| Overview | |
| Safe Harbours | |
| Chapter 2 Exemption for drugs and medicines and goods specifically excluded from exemption | |
| Elements of the exemption | |
| Marketed principally as drugs or medicines | |
| For use in preventing, curing or treating sickness or disease in humans | |
| Exclusion from exemption: toilet preparations | |
| Classes of goods applied topically | |
| Essential oils, herbal and homoeopathic preparations | |
| General rule | |
| Exceptions to the rule | |
| Vitamin E creams and lotions | |
| General rule | |
| Exceptions to the rule | |
| Skin repair creams and lotions | |
| General rule | |
| Exceptions to the rule | |
| Medicated face and skin washes | |
| General rule | |
| Exceptions to the rule | |
| Anti-dandruff hair and scalp treatments | |
| General rule | |
| Exceptions to the rule | |
| Other goods which may be affected by the exclusion | |
| Mouthwashes | |
| General rule | |
| Exceptions to the rule | |
| Exclusion from exemption: medicated confectionery | |
| Throat and cough lozenges | |
| Exclusion from exemption: household disinfectants | |
| Application of other Items | |
| Chapter 3 Safe Harbours | |
| Why provide Safe Harbours? | |
| Rules for using Safe Harbours | |
| Safe Harbours linked to the Therapeutic Goods Act | |
| Safe Harbour 1: Prescription only medicines, controlled drugs and medicines on the Schedule of Pharmaceutical Benefits | |
| Safe Harbour 2: Pharmacy medicine and pharmacy only medicine | |
| Safe Harbour 3: Unscheduled non-prescription non-dermal medicines | |
| Chapter 4 Sunscreen Preparations | |
| Elements of the exemption for sunscreens | |
| Marketed principally for use as a sunscreen preparation for humans | |
| Australian Register of Therapeutic Goods | |
| Categories of sunscreen products | |
| Traditional sunscreen preparations | |
| Multi-purpose preparations | |
| Chapter 5 Date of effect | |
| Application to previous rulings | |
| Public Rulings | |
| Private Rulings | |
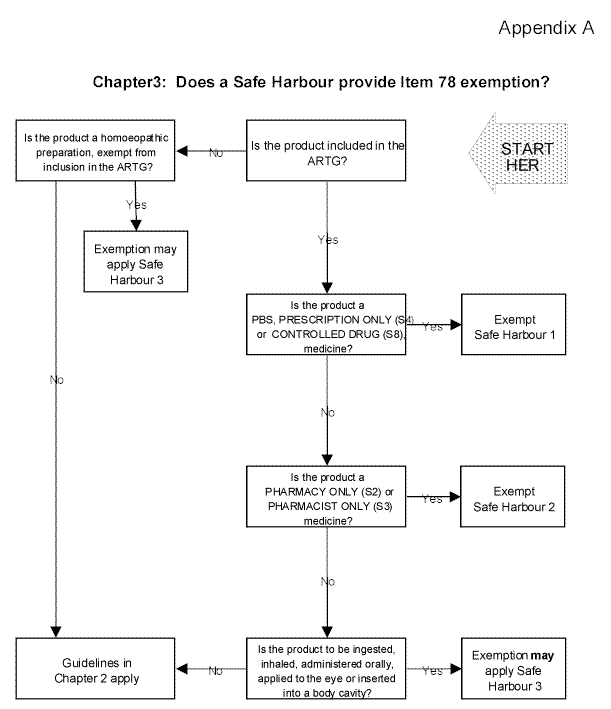
| Appendix A Does a Safe Harbour provide Item 78 exemption? |
| This document is a Ruling for the purposes of section 77 of the Sales Tax Assessment Act 1992 and may be relied upon as if it had the force of law. |
Chapter 1: What this Ruling is about
Overview
1.1 The sales tax law provides exemption from sales tax for certain categories of drugs and medicines and some sunscreen preparations. This Ruling:
- *
- explains the provisions of the law that determine whether products are exempt;
- *
- provides some practical guidelines to make the law easier to apply; and
- *
- explains how manufacturers and importers can get further advice from the Australian Taxation Office (ATO).
1.2 The exemptions, and their exclusions, available for drugs and medicines are discussed in Chapter 2. Chapter 3 sets out some practical guidelines that operate without manufacturers and importers needing to ask the ATO for a private ruling. Chapter 4 deals with sunscreen preparations and Chapter 5 sets out how previous advice from the ATO is affected by this Ruling.
1.3 The principles of interpretation governing the ordinary meaning of words and the essential character of goods are explained in Taxation Ruling SST 11 - Sales tax: a guide to the classification of goods under the sales tax law. The role that marketing plays in determining the classification of some goods is also explained in SST 11. However, because marketing plays a significant role in determining exemption under Items 78 and 91, it is also discussed in this Ruling.
Safe Harbours
1.4 In addition to providing an explanation of the law, this Ruling sets out some practical guidelines to determine if products are exempt drugs or medicines. These guidelines were developed in consultation with industry groups and their representatives. They are termed Safe Harbours and they look like this:
| Safe Harbour |
| A practical test for exemption under Item 78 that the ATO accepts as conclusive. |
1.5 If a product meets the tests of a Safe Harbour it is automatically exempt. It is not necessary to ask the ATO for a private ruling to confirm the exemption. The classification of a product that is exempted by a Safe Harbour does not need to be considered under the general guidelines in Chapter 2 of this Ruling.
1.6 While the Safe Harbours cover several major categories of exempt goods, they don't deal with every product on the market, so manufacturers and importers may still wish to ask for private rulings from the ATO on some products. This may be especially appropriate where a person cannot classify a product under the guidelines set out elsewhere in the Ruling. Penalties may apply if sales tax is not charged correctly.
1.7 This Ruling is expressed in non-technical language wherever possible. The footnotes refer to court decisions on the point, give details of the particular legislation that applies, or dictionary[F1] and publication references as appropriate.
Chapter 2: Exemption for drugs and medicines and goods specifically excluded from exemption
Elements of the exemption
2.1 Item 78 in Schedule 1 to the Sales Tax (Exemptions and Classifications) Act 1992 (ST(E and C) Act) exempts from sales tax:
- (1)
- Goods marketed principally as drugs or medicines for use:
- (a)
- in preventing, curing or treating sickness or disease in humans; or
- (b)
- in compounding or preparing such drugs or medicines.
- (2)
- This Item does not cover:
- (a)
- toilet preparations and goods in the nature of toilet preparations (including soaps, cleansing creams, hair lotions, anti-dandruff foams or shampoos, skin repair creams or lotions, toothpastes, cosmetics, powders, pomades and perfumes);
- (b)
- medicated confectionery;
- (c)
- goods of the following kinds marketed principally as antiseptics:
- (i)
- household disinfectants;
- (ii)
- sterilising solutions;
- (iii)
- combined sterilising solutions and disinfectants;
- (iv)
- combined disinfectants and antiseptics;
- (v)
- combined sterilising solutions, disinfectants and antiseptics.
2.2 Therefore, for goods to be exempt under subitem 78(1)(a) they need to be:
- *
- marketed principally as drugs or medicines; and
- *
- for use in preventing, curing or treating sickness or disease in humans;
- but they must not meet the descriptions of:
- *
- toilet preparations or goods in the nature of toilet pre parations (including the types of goods named in subitem 78(2)(a);
- *
- medicated confectionery; or
- *
- disinfectants.
2.3 The two qualifying elements of the Item and the three categories of exclusion are discussed below.
Marketed principally as drugs or medicines
2.4 Where sales tax exemption depends on how goods are marketed, it is possible for products with common ingredients to be classified differently if they are marketed differently.[F2] For exemption to apply under Item 78, goods must be marketed principally as drugs or medicines. In the sales tax law, principally includes exclusively.[F3] The ordinary meaning of principally is 'in the chief place; above all; pre-eminently'.[F4]
2.5 The term marketed is not defined in the sales tax legislation.[F5] The ordinary meaning of the term is the total process whereby goods are put on the market. With some goods, a number of factors need to be taken into account to determine how they are marketed. This involves consideration on a case by case basis of relevant indicators, none of which may be definitive in its own right. Examples of some indicators are product name, labelling and packaging, advertising, consumer information and point of sale.
2.6 Goods that are marketed principally as something other than drugs or medicines, for example, as cosmetics, do not qualify for exemption under Item 78. In many situations, deciding how goods are marketed principally may prove difficult. The Safe Harbours described in Chapter 3 may be useful in these circumstances.
2.7 A drug is a chemical substance given or taken with the intention of preventing or curing disease, or otherwise enhancing the physical or mental welfare of humans. A medicine is any substance or mixture of substances used in treating or preventing diseases or disorders.[F6]
For use in preventing, curing or treating sickness or disease in humans
2.8 The term for use in does not require the drug or medicine to have proven efficacy, but that it be directed to, or marketed for, the prevention, cure or treatment of sickness or disease.[F7] Item 78 requires a product be marketed principally for therapeutic use. The product may also be marketed for other less significant uses without detracting from exemption.[F8] However, the principal use has to be an appreciable or significant one, not merely a possible or remote use.[F9]
2.9 In the context of the Item, preventing means to ward off disease.[F10] Curing is a method or course of remedial treatment for disease. Treating means dealing with, in order to cure. When read together, the words preventing, curing or treating mean any action taken to stop a person from contracting or spreading a sickness or disease; a course of action taken to make a person, afflicted by a sickness or disease, well; or to relieve the symptoms of sickness or disease.
2.10 Sickness is a particular disease or malady (that is, any bodily disorder). Disease is a morbid or degenerative condition of the body, or of some organ or part. The sickness or disease must be a recognisable condition and not merely a state of general weariness.
Exclusion from exemption: toilet preparations
2.11 Even where goods satisfy the two qualifying elements dealt with above, if they fall within the description of toilet preparations and goods in the nature of toilet preparations they are excluded from exemption by subitem 78(2)(a). The term toilet preparation is not defined in the law so the ordinary or normal meaning applies:
- *
- toilet the act or process of dressing, including bathing, arranging the hair, etc.; the action or process of dressing, or, more recently, of washing or grooming.[F11]
- *
- toiletry an article or substance used in dressing or hygiene, as a soap, deodorant, shaving lotion etc.
- *
- hygiene the science that deals with the preservation of health.
- *
- preparation something prepared, manufactured or compounded.
Accordingly, a toilet preparation can be described as something prepared, manufactured or compounded, for use in the course of dressing, bathing or grooming, or for hygiene.
2.12 In addition to excluding toilet preparations, subitem 78(2)(a) also excludes goods in the nature of toilet preparations and specifies several types of goods that are included in those two expressions:
- *
- soaps;
- *
- cleansing creams;
- *
- hair lotions;
- *
- anti-dandruff foams or shampoos;
- *
- skin repair creams or lotions;
- *
- toothpastes;
- *
- cosmetics;
- *
- powders;
- *
- pomades; and
- *
- perfumes.
In the nature of means having the qualities of.
2.13 The classes of excluded goods given in paragraph 2.12 are marketed for dressing, bathing, grooming and hygiene purposes with a view to protecting or maintaining the body in good condition; cleansing or perfuming the body; or altering the odours of the body. Preparations that provide these attributes include:
- *
- make-up, and hygiene powders;
- *
- perfumes, toilet waters or eau de colognes;
- *
- depilatories;
- *
- antiperspirants and deodorants;
- *
- hair tonics, hair tints or bleaches; and
- *
- shaving creams, foams, lotions and soaps.
2.14 Subitem 78(2)(a) excludes from exemption toilet preparations or goods in the nature of toilet preparations. As a general rule, the exclusion applies to goods that have the essential character of toilet preparations even though the goods may exhibit some medicinal or therapeutic properties.
Classes of goods applied topically
2.15 Products known as 'topicals' are applied dermally, that is, to the skin and scalp. Many topicals are not covered by a Safe Harbour and there may be difficulty in deciding whether or not they are excluded from exemption as toilet preparations. Paragraphs 2.16 to 2.30 discuss the application of subitem 78(2)(a) to several classes of topicals and include a 'general rule' (products caught by the exclusion and therefore likely to be taxable) and an 'exception to the rule' (products not caught by the exclusion and therefore possibly exempt). Products with characteristics that indicate they fall within an exception to the general rule must also meet the tests in subitem 78(1)(a) for exemption to apply. In some instances, where the subitem 78(1)(a) test is met, it is indicated in 'exceptions to the rule'.
Essential oils, herbal and homoeopathic preparations
2.16 Preparations that include volatile essential oils or herbal extracts may be formulated as creams, lotions, oils, gels, ointments or emulsions for application directly or indirectly to external parts of the body. Some essential oils or combinations of oils are mixed with a carrier oil to facilitate application. Jojoba and almond oil are examples of carrier oils. Products consisting of, or containing, essential oils and herbal extracts are used for a wide range of health, beauty and therapeutic purposes. Homoeopathic preparations, often in the form of ointments, may be formulated for the treatment of illnesses that affect the skin.
General rule
2.17 Essential oil and herbal products are excluded from exemption by subitem 78(2)(a) if they are for use on the skin and other parts of the body, such as the hair and eyes, or in the processes of washing and bathing, for purposes including revitalising and rejuvenating the body, deodorising and cleansing, relieving daily tension or tiredness, promoting good health and well being, freshening the breath, and in maintaining healthy hair and scalp. Carrier oils do not qualify for exemption because they are not drugs or medicines.
Exceptions to the rule
- (a)
- Essential oil and herbal products for topical application in the treatment of conditions including nappy rash, muscle sprains and strains, rheumatism, arthritis, cold sores and rashes, cracked nipples, upset stomach and headache, are not caught by excluding subitem 78(2)(a) where it can be demonstrated that the product is directed to these and other medical conditions. For example, a product may be applied to the skin so the active ingredient can be absorbed into the bloodstream. It must also be demonstrated that any toilet or cosmetic attributes of the product are of a secondary or adjunctive role to the therapeutic role. In these cases the tests required to satisfy subitem 78(1)(a) are usually met.
- (b)
- Homoeopathic preparations for the treatment of cuts or similar conditions are not caught by the exclusions. They also satisfy subitem 78(1)(a).
Vitamin E creams and lotions
2.19 Vitamin E creams and lotions have many uses, including softening, soothing or protecting the skin, treating minor irritations and helping in the healing of cuts and abrasions. In some cases, Vitamin E creams are used in treating mild sunburn and minor burns.
General rule
2.20 Vitamin E products are excluded from exemption by subitem 78(2)(a) if they are for use to enrich, sooth or soften dry or chafed skin and for similar purposes. These products are taxable.
Exception to the rule
2.21 Vitamin E products for use in treating illnesses and conditions such as burns, abrasions, nappy rash, cuts, wounds and other injuries, are not excluded from exemption by subitem 78(2)(a), where it can be demonstrated that the product is directed to the treatment of these or other medical conditions. It must also be demonstrated that any skin repair, toilet or cosmetic attributes of the product are of a secondary or adjunctive role to the therapeutic role. These products meet the marketed principally test in subitem 78(1)(a) if they are not promoted for uses described in the general rule or if those uses, so described, do not give the products the character of a toilet preparation.
Skin repair creams and lotions
2.22 While some skin repair creams and lotions may contain herbal products, essential oils or Vitamin E, many are formulated without these ingredients. These products are frequently used for health and beauty purposes.
General rule
2.23 Skin repair products are used for purposes that include moisturising dry or normal skin, cleansing, revitalising, and rejuvenating the skin and body. These and similar uses point to the products having the character of either skin repair creams and lotions or cosmetics. The specific exclusion in subitem 78(2)(a) applies wherever a product has the character of a skin repair cream or lotion. These products are taxable.
Exception to the rule
2.24 A product must have a character other than that of a skin repair cream to escape the specific subitem 78(2)(a) exclusion. A product that appears to be a skin repair cream beca use it has moisturising and other skin repair attributes but is for use in treating conditions including nappy rash, eczema, cold sores, rashes or cracked nipples, escapes the exclusion if it can be demonstrated that the latter therapeutic uses dominate its character.
Medicated face and skin washes
2.25 Medicated face and skin washes comprise a range of products presented in liquid, lotion, foam or bar form. They are generally used to clean the skin, in some cases as a preparatory step to the application of an anti-acne product. Washes are frequently described as being able to cleanse deep into the pores of the skin to remove dirt and excessive oil. Face and skin washes frequently use the term 'medicated' to indicate the inclusion of an antiseptic additive.
General rule
2.26 Products that predominantly perform a cleansing, washing or moisturising action, sometimes for use with sensitive skin, are caught by the subitem 78(2)(a) exclusion and are taxable. The exclusion applies regardless of whether the product contains an anti-bacterial agent or another active therapeutic ingredient.
Exception to the rule
2.27 Products that treat or prevent a specific medical condition, for example, acne, are not caught by excluding subitem 78(2)(a) where any cleansing or moisturising characteristic of the product acts as either an adjunct to the treatment or is the vehicle by which the product can achieve its desired effect. These products also meet the subitem 78(1)(a) tests and are exempt.
Anti-dandruff hair and scalp treatments
2.28 Anti-dandruff treatments, generally in liquid or foam forms, are used to clean the hair and scalp and to remove dead skin. Other scalp treatments are directed at fungal complaints often associated with serious illnesses.
General rule
2.29 Anti-dandruff foams and shampoos, where the principal actions are to remove dandruff and to cleanse the scalp and hair, are included in the list of goods caught by excluding subitem 78(2)(a). These products are taxable.
Exceptions to the rule
- (a)
- Products for use in treating the more severe underlying fungal infections (usually at the recommendation of a medical practitioner) are not caught by the subitem 78(2)(a) exclusion, where it can be demonstrated that the purpose of any shampoo or foam action is to facilitate the effectiveness of the active ingredient, for example, by delivering the active ingredient to the target area. These products satisfy the subitem 78(1)(a) tests and are exempt.
- (b)
- Products for use in treating head lice are not caught by the subitem 78(2)(a) exclusion, where it can be demonstrated that any shampoo or foam action is for the purpose of enabling the active ingredient to be applied to the hair or scalp and any cleaning action is incidental to the therapeutic purpose. These products satisfy the subitem 78(1)(a) tests and they are exempt.
Other goods which may be affected by the exclusion
Mouthwashes
2.31 There are two main types of mouthwash that are marketed as having antiseptic or anti-bacterial properties, namely, breath fresheners and mouthwashes. Breath fresheners assist the user to achieve fresh breath by removing loose debris in the mouth and masking breath odours. Other mouthwashes are designed to treat medical conditions such as mouth infections and ulcers or are used in conjunction with dental treatment.
General rule
2.32 Breath fresheners and mouthwashes used to mask breath odours and remove loose debris from the mouth are excluded from exemption as toilet preparations (even if one of their uses could be considered to be in treating or preventing sickness or disease) and are therefore caught by the subitem 78(2)(a) exclusion. These products are taxable.
Exception to the rule
2.33 Mouthwashes designed to treat or remove plaque as an aid in the treatment of gingivitis, as antiseptics for mouth disorders, or in conjunction with dental treatment are not caught by the subitem 78(2)(a) exclusion, provided it can be demonstrated that any cleansing or masking action is incidental to the therapeutic role and facilitates the effectiveness of the active ingredient in the treatment.[F12] These products meet the subitem 78(1)(a) tests and are exempt.
Exclusion from exemption: medicated confectionery
2.34 Medicated confectionery is excluded from exemption by subitem 78(2)(b). The term medicated confectionery is not defined in the sales tax law so the ordinary or normal meaning applies:
- *
- medicate impregnate with a medical substance;
- *
- confection a sweet or bonbon;
- *
- confectionery things made or sold by a confectioner.[F13]
2.35 Considering the above dictionary meanings in context, the ordinary or normal meaning of medicated confectionery can be said to be sweet preparations formulated to include one or more medical substances. These preparations are often marketed in the form of lozenges or drops.
Throat and cough lozenges
2.36 Many types of throat or cough lozenges or drops have the essential character of medicated confectionery and are therefore excluded from exemption. The ingredients of these goods include those found in ordinary confectionery (e.g., glucose and gum). Other ingredients normally found in medicated confectionery, that is, eucalyptus or menthol, are often present. Marketing terminology uses terms synonymous with 'soothing action'.
2.37 Other throat or cough lozenges exhibit characteristics that preclude them from being known as medicated confectionery. These lozenges qualify for exemption under subitem 78(1)(a). The characteristics are that the products are marketed:
- *
- with active ingredients not normally found in medicated confectionery (e.g., anti-bacterial substances);
- *
- to treat a sickness or disease (e.g., coughs or sore throats); and
- *
- with dosage requirements on their labelling.
Exclusion from exemption: household disinfectants
2.38 Subitem 78(2)(c) is the third category of exclusion from exemption as drugs or medicines. The exclusion covers the following kinds of goods:
- *
- household disinfectants;
- *
- sterilising solutions; and
- *
- combinations of disinfectants, sterilising solutions and antiseptics.
2.39 Antiseptic derives from the noun antisepsis. Antisepsis is defined to mean the destruction of micro-organisms that produce sepsis or septic disease. An antiseptic can be described as a substance that is intended for application to the skin or mucous membranes, to kill or prevent the growth of bacteria on the human body.
2.40 Disinfectants are used to control the spread of infection by killing the bacteria responsible. They are normally used to wash floors, walls and toilets in houses and public buildings, or are added to water to wash clothing or babies' nappies. Sterilising solutions are used to kill microbes and to stop the reproduction of microbes on inanimate objects and surfaces. Terms are relevantly defined as:
- *
- disinfect to cleanse (rooms, clothing, etc.) from infection; destroy disease germs;
- *
- disinfectant any chemical agent that destroys bacteria;
- *
- sterilise to destroy micro-organisms.
2.41 The exclusion applies to products that may have antiseptic uses and are of a kind normally used for killing bacteria or preventing its spread on surfaces and objects other than the human body (including cleaning to prevent contamination). The goods excluded are straight disinfectants, sterilisers and combinations of these goods, including antiseptic combinations. However, antiseptics for application to the skin or for taking orally, are not affected by the exclusion, even if they may also be capable of being used as disinfectants.
Application of other Items
2.42 Goods that are clearly excluded from exemption as drugs or medicines under Item 78 are not generally exempted by Items 80 and 93, which deal respectively with medical and surgical goods and goods for disabled persons.
2.43 Item 80 exempts from sales tax:
- Medical or surgical goods of a kind ordinarily used by persons suffering from a medical condition where that use is for the purpose of alleviating or treating the condition or its effects.
- [Parts]
- In this Item, 'medical condition' means sickness, disease, injury or physical impairment.
2.44 Item 93 exempts from sales tax:
- Goods that:
- (a)
- are designed and manufactured expressly for use by persons who suffer from sickness, disease or disablement; and
- (b)
- are of a kind not ordinarily used by persons who do not suffer from sickness disease or disablement.
- In this Item, 'disablement' includes blindness or deafness.
- [Parts]
2.45 Goods that fall for classification under Item 78 are distinct from those covered by Items 80 or 93. Item 78 provides exemption for goods that are strictly consumables, while Items 80 and 93 are concerned with goods that are generally appliances or equipment and some related consumables.
2.46 Eye droppers, medicine glasses and hearing aids are examples of goods that are exempt under Item 80. Artificial limbs and orthotic devices are examples of goods exempt under Item 93. Specific use consumable goods that enable the use of other exempt goods, like comfort drops for wearers of contact lenses, are also exempt under Item 93.
Chapter 3: Safe Harbours
Why provide Safe Harbours?
3.1 Several tests are required to determine whether a product is exempt from sales tax under Item 78. Each test may involve a degree of complexity, needing the exercise of some judgment. However, there are many goods that are so obviously drugs or medicines and not cosmetics, toilet preparations or other types of excluded goods, that a formal consideration of each step involved in determining exemption seems hardly necessary.
3.2 In an area such as this, where there are potentially many thousands of products to be classified and the legislative tests are difficult, we use Safe Harbours as a way to provide certainty and market neutrality for taxpayers. The Safe Harbours remove the formal consideration otherwise necessary to determine exemption for many, but not all, of the goods that could be considered exempt under subitem 78(1).
3.3 A person who acts reasonably, in reliance of a Safe Harbour has the protection of section 77 of the Sales Tax Assessment Act 1992.[F14]
Rules for using Safe Harbours
3.4 If a product meets the tests of a Safe Harbour then it is exempt under Item 78. There is no need to ask the ATO for a private ruling to confirm the exemption. If a Safe Harbour is subsequently changed, the change only affects a taxpayer prospectively. Safe Harbours apply prospectively from the date of effect of this Ruling and nothing in them can be taken to give rise to a credit entitlement in respect of the past. However, a person who believes they have overpaid tax before the date of effect of this Ruling may apply for a refund and the a pplication w ill be dealt with on the basis of the general principles contained in this Ruling. Failure to meet the tests of a Safe Harbour does not mean that a product is taxable, only that classification needs to be considered in the light of the principles explained in Ch apter 2.
3.5 If a manufacturer or importer is uncertain as to whether a Safe Harbour applies to particular goods, that person may seek guidance from the ATO by requesting a private ruling. Alternatively, if they consider that exemption applies, but the goods are not covered by a Safe Harbour, they may also ask for a private ruling. The Appendix to this Ruling contains a flow-chart that may be used as a guide to whether a Safe Harbour applies.
Safe Harbours linked to the Therapeutic Goods Act
3.6 As explained in Chapter 2, one of the requirements to be met for exemption under Item 78 is that the goods must satisfy a marketed principally test. Determining how goods are marketed principally can be difficult and manufacturers and importers have sought a simpler way to determine this, based on concepts more familiar to them.
3.7 The marketing of drugs and medicines in Australia is subject to the requirements of therapeutic goods legislation administered by the Therapeutic Goods Administration (TGA), an arm of the Commonwealth Department of Health and Family Services. The regulation of therapeutic goods in Australia is substantially governed by the Therapeutic Goods Act 1989 and the Therapeutic Goods Regulations.[F15] Under therapeutic goods legislation, products making therapeutic claims or containing prescribed substances must be included in the Australian Register of Therapeutic Goods (ARTG), unless exempt from inclusion or otherwise specifically approved.[F16] The ARTG is divided into two categories - registrable and listable goods. Schedules to the Regulations generally govern the relevant applicable category.
3.8 The therapeutic goods legislation and the Therapeutic Goods Advertising Code[F17] impose a marketing test on products that make therapeutic claims. Unlike sales tax exemption Item 78, there is no requirement that a product's therapeutic uses must be the principal purpose for which it is marketed. Another important difference is that the sales tax law does not exempt products that are toilet preparations, cosmetics, etc., regardless of whether or not therapeutic claims are made for these products.
3.9 Despite the different marketing tests applicable to the therapeutic goods legislation and Item 78, the ATO recognises that classification can be difficult without some practical industry benchmark or guide. We have therefore decided to use the ARTG for this purpose in formulating each Safe Harbour. If a product is not included in the ARTG, it cannot have access to a Safe Harbour and its classification must be considered under the general guidelines set out in Chapter 2. An exception applies to those homoeopathic preparations covered by Safe Harbour 3 that are exempt from inclusion in the ARTG. Products that are included in the ARTG but not covered by a Safe Harbour also need to be classified under the Chapter 2 guidelines.
Safe Harbour 1: Prescription only medicines, controlled drugs and medicines on the Schedule of Pharmaceutical Benefits
3.10 Some drugs and medicines contain substances that are included in Schedule 4 (S4) or Schedule 8 (S8) of the Standard of the Uniform Scheduling of Drugs and Poisons (SUSDP).[F18] An S4 or S8 medicine may be referred to as a prescription only medicine or a controlled drug respectively. This is because these 'signal' words must appear on the labelling of S4 and S8 medicines before they may be sold or supplied for human use.[F19] A prescription must also be given by a medical or dental practitioner before a prescription only medicine or controlled drug may be sold for human use. Safe Harbour 1 exempts these goods.
3.11 Safe Harbour 1 also exempts drugs and medicines included in the Schedule of Pharmaceutical Benefits (PBS). prescription only medicine, controlled drug and PBS drugs and medicines are required to be included in the ARTG.
| Safe Harbour 1 - Item 78, Schedule 1 |
| Goods are drugs or medicines for human use and exempt under Item 78 if they are: |
| (1) a prescription only medicine (S4) |
| (2) a controlled drug (S8); or |
| (3) listed in the PBS (as published from time to time). |
Safe Harbour 2: Pharmacy medicine and pharmacy only medicine
3.12 Some drugs and medicines contain substances included in Schedule 2 (S2) or Schedule 3 (S3) of the SUSDP. An S2 or S3 medicine may be referred to as a pharmacy medicine or a pharmacist only medicine respectively . This is because these 'signal' words are required to be shown on the labelling of S2 and S3 medicines before they may be sold or supplied.[F20] The assistance of a pharmacist should be available if a person needs advice when buying a pharmacy medicine. A pharmacist should be involved in the supply of a pharmacist only medicine. S2 and S3 medicines must be included in the ARTG. Safe Harbour 2 exempts these goods.
| Safe Harbour 2 - Item 78, Schedule 1 |
| Goods are drugs or medicines for human use and exempt under Item 78 if they are a pharmacy medicine (S2) or a pharmacist only medicine (S3). |
Safe Harbour 3: Unscheduled non-prescription, non-dermal medicines
3.13 Safe Harbour 3 exempts two groups of drugs and medicines that are neither included in the SUSDP (under Schedules 2, 3, 4, or 8) nor require a prescription from a medical or dental practitioner before they may be sold. The first group covers those medicines that are swallowed, ingested, inhaled or administered orally for systemic action but does not include medicated confectionery, toothpastes, mouthwashes and mouthrinses or products excluded from exemption by subitem 68(2) in Schedule 1. The second group covers those drugs or medicines that are to be applied to the eye's surface or inserted into body cavities (for example, aural, nasal and vaginal) but does not include products marketed for use in connection with sexual activity or personal hygiene. To be covered by Safe Harbour 3 these goods must be included in the ARTG.
3.14 Exemption under Safe Harbour 3 also extends to homoeopathic preparations included in the ARTG or exempt from inclusion under Schedule 5 to the Therapeutic Goods Regulations.
3.15 In practice, this Safe Harbour exempts, among other things, the following products if they are included in the ARTG:
- *
- vitamin and mineral tablets;
- *
- vitamin and mineral preparations to be mixed with water, milk or fruit juice; and
- *
- herbal and some essential oil therapeutic products for non-dermal use.
However, prepared beverages, or ingredients for beverages to be mixed with water, milk or fruit juice, are not covered by this Safe Harbour if they are for the general nourishment or enjoyment of normally healthy persons.[F21]
| Safe Harbour 3 - Item 78, Schedule 1 |
| Goods are drugs or medicines for human use and exempt under Item 78 if they are included in the ARTG and are: |
| (1) to be swallowed, ingested, inhaled or administered orally for systemic action, |
| but are not medicated confectionery, toothpastes, mouthwashes and mouthrinses, or products excluded from exemption by subitem 68(2); |
| (2) applied to the surface of the eye or inserted into body cavities (including aural, nasal and vaginal), |
| but are not goods for use in connection with sexual activity or personal hygiene. |
| Goods are also drugs or medicines for human use and exempt under Item 78 if they are: |
| (3) homoeopathic preparations to be taken as drops or pilules and are exempt from inclusion in the ARTG under Item 8(a) of Schedule 5 to the Therapeutic Goods Regulations. |
Chapter 4: Sunscreen preparations
Elements of the exemption for sunscreens
4.1 Item 91 in Schedule 1 to the ST(E and C) Act exempts from sales tax:
- Goods marketed principally for use as sunscreen preparations for humans and included in the Australian Register of Therapeutic Goods.
4.2 Therefore, for goods to be exempt under Item 91, they need to be:
- *
- marketed principally for use as a sunscreen preparation for humans; and
- *
- included in the ARTG.
4.3 The two qualifying elements of the Item are discussed below.
Marketed principally for use as a sunscreen preparation for humans
4.4 Principally means 'in the chief place; above all; pre-eminently' and is defined in the sales tax law to include exclusively (see paragraph 2.4). The manner in which sunscreen products are marketed needs to be determined in accordance with the guidelines set out in paragraphs 2.4 and 2.5. Some further comments on marketing are provided in paragraphs 4.8 to 4.16.
4.5 A sunscreen is a cream or lotion applied to the skin to screen it from ultraviolet rays and prevent sunburn.[F22] A preparation is something prepared, manufactured or compounded. A sunscreen preparation is a preparation applied to the skin to reduce the incidence of skin damage caused by exposure to the sun. Sunscreen preparations may also be presented for use in other forms, for example, gels, balms and mousses.
Australian Register of Therapeutic Goods
4.6 Where a product states on its label that it has a sun protection factor (SPF) of 4 or greater it must be included on the ARTG. A sunscreen that is included on the ARTG must show, on its labelling, its listing or registration number. Listed numbers are prefaced with AUST L, registered numbers are prefaced with AUST R.[F23]
4.7 The Therapeutic Goods Administration issues a certificate verifying that a sunscreen preparation is included on the ARTG. This certificate is evidence that the ARTG test in Item 91 has been met. Any questions concerning the requirements of the Therapeutic Goods Administration should be directed to the Administration's Information Officer.[F24]
Categories of sunscreen products
4.8 Paragraphs 4.9 to 4.16 provide guidelines to assist in determining the marketed principally test for several categories of products that have SPF ratings.
Traditional sunscreen preparations
4.9 Traditional sun protection products provide sun protection for persons engaged in many outdoor activities. Examples of activities include sunbaking, swimming, sailing, playing sport and working in the field. Traditional products are generally supplied in cream, lotion, milk and mousse formulas.
4.10 Some features common to traditional sunscreen preparations are:
- *
- a name that makes sun protection prominent, by including words like 'Sunscreen Cream', 'Sunscreen Lotion', or 'Blockout';
- *
- an SPF rating clearly shown on the label; and
- *
- the inclusion of directions for use, for example, 'Reapply every 2 hours', 'Apply 20 minutes before going out into sun' and 'Reapply if wiped with towel'.
4.11 A traditional sunscreen protection product me ets the tests of Item 91 where the product is included in the ARTG and it can be demonstrated that the product has either no cosmetic attributes or any cosmetic attributes are not marketed above the sun protection attribute.
Multi-purpose preparations
4.12 In response to shifting c onsumer expectations, multi-purpose products have been developed for use on the skin, particularly the face, neck and hands. These products are sometimes referred to as secondary sunscreens. They promote cosmetic benefits and provide a level of sun protection appropriate for less extreme conditions or transient exposure to the sun. Examples of transient exposure include going to and from indoor work, doing the shopping, spending a short time outdoors and picking up the children from school.
4.13 Some multi-purpose preparations are marketed under well known cosmetic brand names. The degree of effect, if any, an established cosmetic brand name has on a product's marketing is a question that must be considered together with other relevant marketing factors. Directions for use provided for multi-purpose products are another marketing consideration. The absence of certain directions seen on traditional sunscreen preparations, for example, 'Reapply if wiped with towel', do not preclude a product from exemption. These directions are generally inappropriate for multi-purpose products and, as a general rule, are not included on labelling. However, other directions explaining how and when to apply a product are relevant to the question of how a product is marketed.
4.14 The presence of SPF wording in a product's labelling, in itself a relevant marketing consideration, does not necessarily mean the product satisfies the marketed principally test of Item 91.
4.15 There is also a range of sunscreen products marketed for specific application to the lips. Many products in this range are multi-purpose. For exemption to apply, these products must satisfy the two tests contained in Item 91.
4.16 In determining whether a multi-purpose lip product is marketed principally as a sunscreen preparation, a reference to cold or wind protection, moisturising qualities, or the addition of colourings or flavours, does not necessarily prevent the product from qualifying for exemption but may be relevant when considered in conjunction with other marketing factors.
4.17 If it can be demonstrated that a multi- purpose product is a sunscreen preparation with cosmetic benefits, rather than a cosmetic or toilet preparation with sun protection benefits, the Item 91 marketed principally test is met.
Chapter 5: Date of effect
5.1 This Ruling is effective immediately. Nothing in this Ruling may be taken as automatically authorising a refund before the date of effect of the Ruling. Credit claims are considered on their individual merits.
5.2 The effect of this Ruling on existing public and private rulings is discussed in the following paragraphs.
Application to previous rulings
Public Rulings
5.3 The following Rulings are withdrawn[F25]:
- ST 2041; ST 2048; ST 2078; ST 2250; ST 2251 and ST 2423.
Private Rulings
5.4 Previous private rulings that are inconsistent with the principles in this Ruling will be altered from 12 November 1998.
5.5 Private rulings on the classification of drugs and medicines apply for 5 years from the date they are issued (or such shorter period requested by the taxpayer), provided the facts on which they were based remain the same. During that period, the ruling may be reviewed at the instigation of either the taxpayer or the ATO and altered by a subsequent ruling. At the end of the period, the ruling lapses if not renewed. For information about what to do if a private ruling lapses, see paragraphs 5.5 and 5.6 of Taxation Ruling SST 11 'Sales tax: a guide to the classification of goods under the sales tax law'.
Commissioner of Taxation
12 August 1998
Footnotes
1. Definitions included in the Ruling are from The Macquarie Dictionary unless otherwise indicated.
2. See the remarks of Lord Reid in Customs and Excise Commissioners v. Beecham Foods Ltd [1972] 1 WLR 241 at 243-244.
3. Section 3(2) of the ST(E and C) Act.
4. Definition in The New Shorter Oxford English Dictionary.
5. For a discussion of the term in a sales tax context, see Case 10/97 97 ATC 167 at 171; AAT Case 11,643 (1997) 35 ATR 1001 at 1005.
6. See the comments of Lockhart J in Bristol-Myers Company Pty Limited v. FCT 90 ATC 4553 at 4558; (1990) 21 ATR 417 at 423 and the comments of Davies J in Parke Davis Pty Ltd v. FCT & Anor 96 ATC 4865 at 4868; (1996) 33 ATR 463 at 467.
7. See comments of Davies J in Parke Davis Pty Ltd v. FCT & Anor 96 ATC 4865 at 4871; (1996) 33 ATR 463 at 469.
8. cf restrictions placed on advertising of therapeutic goods by the Therapeutic Goods Advertising Code.
9. See DFC of T v. Stewart (1984) 154 CLR 385 at 401; 84 ATC 4146 at 4155; (1984) 15 ATR 387 at 398.
10. Antiseptics to be applied directly to cuts and abrasions, etc., prevent infection and therefore ward off disease.
11. The dictionary definitions were adopted by Davies J in Parke Davis Pty Ltd v. FCT & Anor 96 ATC 4865 at 4878; (1996) 33 ATR 463 at 476.
12. For an example of a mouthwash that is not a toilet preparation see Parke Davis Pty Ltd v. FCT & Anor 96 ATC 4865; (1996) 33 ATR 463.
13. Definitions of 'medicate' and 'confectionery' from The New Shorter Oxford Dictionary.
14. Section 77 provides that where a person acts in reliance of a public sales tax ruling, any tax they may have underpaid in reliance on that ruling is remitted.
15. The Therapeutic Goods Act 1989 provides a uniform national system of control over therapeutic goods. However, some limitations apply. Complementary State legislation is required to cover unincorporated parties that trade within one State or Territory (to date, only NSW and Victoria have introduced such legislation).
16. See section 4(1) of the Therapeutic Goods Act 1989.
17. The Therapeutic Goods Advertising Code of the Media Council of Australia is the basis of the advertising regulations under the Therapeutic Goods Act 1989.
18. The Standard is the recommendation of the National Health and Medical Research Council.
19. See Division 1 of Part 2 of the Standard. The labelling requirement took effect from 21 September 1997. Prior to this date, S4 and S8 product labelling included the words 'Caution S4' or 'Caution S8'.
20. See Division 1 of Part 2 of the Standard. The labelling requirement took effect from 21 September 1997. Prior to this date, S2 and S3 product labelling included the words 'Poison S2', 'Caution S2' or 'Caution S3'.
21. Examples of excluded preparations are 'Staminade' and 'Sustagen Gold' (see Nicholas Kiwi Pty Ltd v. FC of T 90 ATC 4662; (1990) 21 ATR 686 and Bristol-Myers Co Pty Ltd v. FC of T 90 ATC 4553; (1990) 21 ATR 417).
22. Definition from the New Shorter Oxford English Dictionary.
23. The ARTG is divided into two categories: registrable and listable goods. Schedules to the Therapeutic Goods Regulations detail the relevant category that applies.
24. The postal address of the Therapeutic Goods Administration is PO Box 100, WODEN, ACT, 2606.
25. Note that the following Sales Tax Rulings were withdrawn at the time of the introduction of the Streamlined Sales Tax Law: ST 2066; ST 2085; ST 2160; ST 2252; ST 2301; ST 2395; ST 2414.
Previously released to the public in (unnumbered) draft form on 16 March 1995
and as SST D12 on 21 January 1998
References
ATO references:
NO NAT 98/24-8
Related Rulings/Determinations:
ST 2041
ST 2048
ST 2078
ST 2250
ST 2251
ST 2423
Subject References:
classification of goods, drugs and medicines, sunscreen products, sales tax
Legislative References:
Sales Tax Assessment Act 1992 Section 77
STEC Act 1992 paragraph 68(2) Schedule 1
STEC Act 1992 Item 78 Schedule 1
STEC Act 1992 subitem 78(1) Schedule 1
STEC Act 1992 subitem 78(1)(a) Schedule 1
STEC Act 1992 subitem 78(2)(a) Schedule 1
STEC Act 1992 subitem 78(2)(b) Schedule 1
STEC Act 1992 subitem 78(2)(c) Schedule 1
STEC Act 1992 Item 80 Schedule 1
STEC Act 1992 Item 91 Schedule 1
STEC Act 1992 Item 93 Schedule 1
Therapeutic Goods Act 1989 4(1)
Therapeutic Goods Regulations
Case References:
Bristol-Myers Company Pty Limited v. FCT
90 ATC 4553
(1990) 21 ATR 417
Customs and Excise Commissioners v. Beecham Foods Ltd
[1972] 1 WLR 241
DFC of T v. Stewart
(1984) 154 CLR 385
84 ATC 4146
(1984) 15 ATR 387
Nicholas Kiwi Pty Ltd v. FCT
90 ATC 4662
(1990) 21 ATR 686
Parke Davis Pty Ltd v. FCT & Anor
96 ATC 4865
(1996) 33 ATR 463
Case 10/97
97 ATC 167
AAT Case 11,643
(1997) 35 ATR 1001
